Chemical and Biological Characterization of Oleanane Triterpenoids from Soy
Abstract
:Introduction
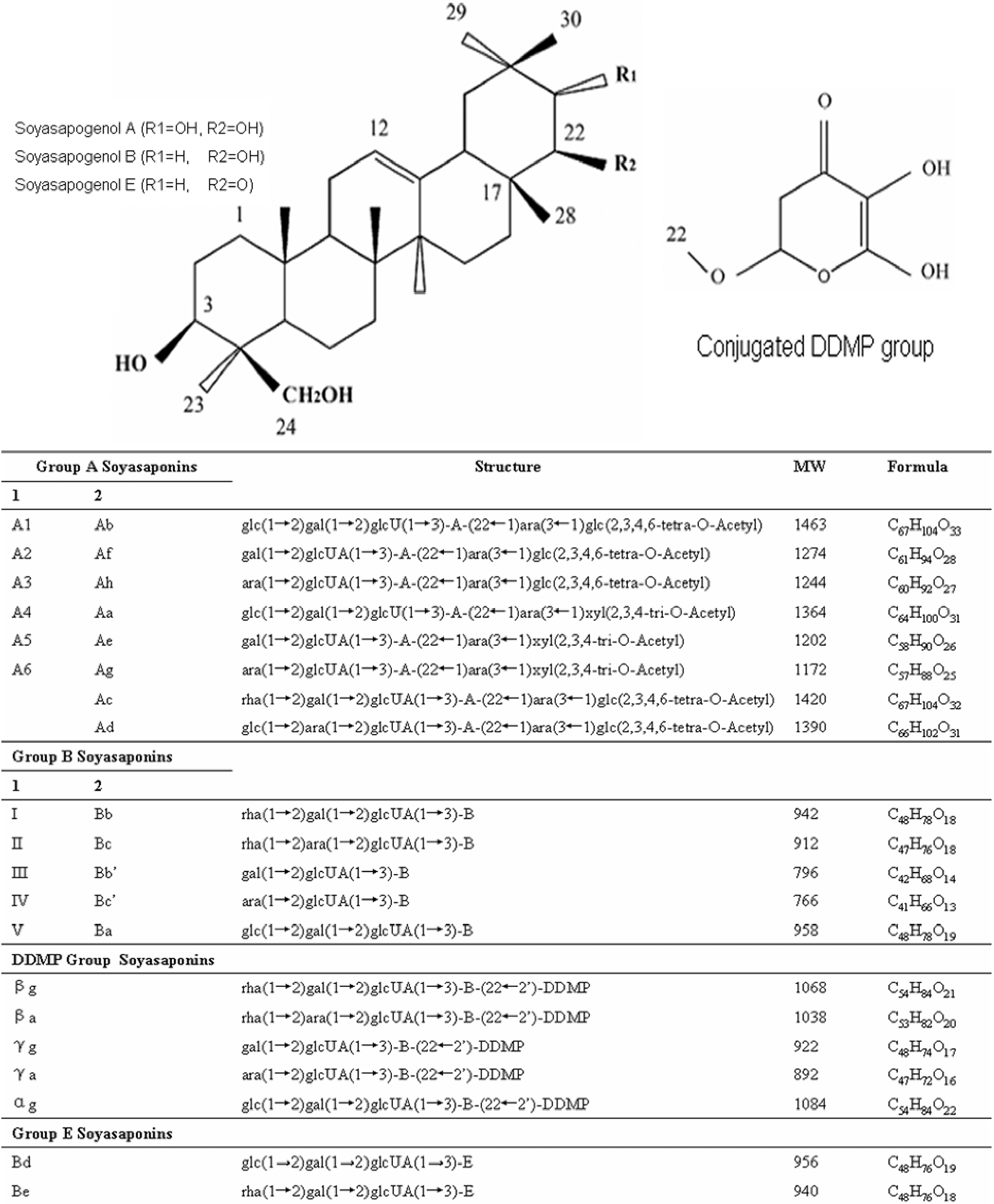
Soyasaponin classification
Soyasaponin extraction
Soyasaponin extract preparation
Analysis and determination of soyasaponins
| Concentration Method [ref.] | Group A | Group B | Column | Group A Solvent Program | Group B Solvent Program |
|---|---|---|---|---|---|
| XAD-2 [29] | Ab, Non-acetylated Ab, | Ba, Bb, αg, βg | Source 15 RP | Gradient A: Water with 0.001% acetic acid (v/v); B: Acetonitrile with 0.001% acetic acid (v/v) | Gradient A: Water with 0.001% acetic acid (v/v); B: Acetonitrile with 0.001% acetic acid (v/v) |
| Silica gel [22] | All | All | Semi-Preparative Waters íBondapak C18 column | Isocratic Methanol, 2-propanol, water and formic acid (45:5:50:0.1) (v/v) | Isocratic Methanol, 2-propanol, water, and formic acid (55:5:40:0.1) (v/v) |
| Flash chromatography system [31] | All | All | Preparative HPLC system Luna C18(2) column | Pre-equilibrated Acetic acid, acetonitrile, and water (1:30:69) (v/v). Gradient A: 100% Acetonitrile B: Water | Pre-equilibrated Acetic acid, acetonitrile, and water (1:30:69) (v/v). Gradient A:100% Acetonitrile B: Water |
| SPE [15,19] | All | All | SPE | Water and Methanol | Water and Methanol |
| C18 Lobar column [18] | NA | All | Semi-preparative HPLC systemRP-18 column | Isocratic DDMP: Acetonitrile, water, TFA (40:59.95:0.05) (v/v) NON-DDMP: Acetonitrile, water, TFA (36:63.95:0.05) (v/v) | |
| C18 Cartridge [32] | NA | Ba and Bb in human serum | HPLC-MS system In MRM mode XDB-C18 column | Gradient A: 0.025% AcOH in water (v/v); B: 0.025% AcOH in MeCN (v/v) |
| Compound | MW | Formula | Analysis Method | Solvent System and Program | Specification |
|---|---|---|---|---|---|
| Soyasapogenol A | 474 | C30H50O4 | TLC [33] Silica gel 60G | Light petroleum (b.p. 60-80 °C), ethyl acetate (4:3) (v/v) | Visualization 10% sulfuric acid in ethanol and viewing under UV |
| TLC [34] | Dichloromethane and methanol (9:1) (v/v) | Spraying with a saturated solution of potassium dichromate in sulfuric acid | |||
| Normal HPLC [33] | A: Light petroleum (b.p. 60-80°C); B: Ethanol, 0-7.5min, 0-7.5% B; 7.5-15 min, 7.5% B isocratic; 15-20 min, 7.5-20% B | Silica Column (250mm × 4.6mm) Flow-rate 1.5 mL/min | |||
| Soyasapogenol B | 458 | C30H50O3 | Revised HPLC [12] | Acetonitrile: 1-propanol: water: 0.1% acetic acid (80:6:13.9:0.1) (v/v) Isocratic | ODS C18 column (250mm × 4.6mm) Flow-rate 0.9mL/minELSD detection |
| Revised HPLC [35] | A: Acetonitrile: 1-propanol: water: acetic acid (80:6:13:0.1) (v/v); B: 100% Acetonitrile 0-15 min 100% A isocratic; 15-17 min 0-100% B; 17-19 min 100% B; 19-22 min back to 100% A | RP-C18-AB column (250 × 4.6 mm) Flow-rate 0.9 mL/min |
| LC-MS System | LC-MS LC Program | MS Condition | LC-MS Mode | Detected Soyasaponins |
|---|---|---|---|---|
| Agilent 1100 series LC/MSD Trap SL [39] | Waters AccQ.Tag column A: 0.025% AcOH in water (v/v) B:0.025% AcOH in MeCN (v/v) temperature: 35°C flow-rate: 0.5 mL/min | ESI Negative mode Capillary voltage: 4.4 Kv Dry Temperature: 350 °C | MRM Transition Setting m/z: 958→940, 942→924 and 822→351 | Ba, Bb in serum |
| Waters/Micromass Ultima LC/MS instrument, consisting of Waters 2690 liquid chromatograph with a Waters 996 PDA [40] | Zorbax Eclipse XDB-C18 column A: Water with 0.05% TFA (v/v) B: ACN with 0.05% TFA (v/v) flow-rate: 0.5 mL/min | ESI Positive mode Capillary voltage: 3.5 Kv Dry Temperature: 350 °C | Full Scan SIR quantification | Group A: Ab, Ac, Af Deacetyl Ab, Ac, Af Di-deacetyl Ab, Tetra-deacetyl Ab, Af Tri-deacetyl Ad Group B: Ba, Bb, Bb′, Bc, Bc DDMP Bb, Bc, Ba |
| Agilent LC/MSD Trap SL [32] | Waters AccQ.Tag column A: 0.025% acetic acid in water (v/v) B: 0.025% acetic acid in MeCN (v/v) temperature: 35 °C flow-rate: 1 mL/min | ESI Negative mode Capillary voltage: 4.4 Kv Dry Temperature: 350 °C | Full Scan SIR quantification | Group A: Aa-Af Group B: Bb, Bb’, Bd and Be DDMP αg, βg, βa, γg |
| Agilent 1100 series LC/MSD Trap SL [30] | The same as [39] | ESI Negative mode Capillary voltage: 4.4 Kv Dry Temperature: 350°C | Full Scan SIR quantification | Group A: Aa, Ab Group B: Ba, Bb, Bb’DDMP βg |
| Waters HPLC with Finnigan LCQ quadrupole ion trap MS with MSn [19] | Shimadzu reversed phase C-18 A: 2.5% acetic acid in water (v/v) B: 100% Acetonitrile Column temperature: 25 °C flow-rate:1 mL/min | ESI Positive and Negative Capillary voltage: 4.4 Kv Dry temperature: 200 °C | Full Scan | Group B: I, III, DDMP βg, βa, γg, γa Group E: Be |
| Bruker Esquire LC with ESI-MS system [41] | J.T.Baker C18 reverse column Linear solvent: 0.1% acetic acid in water/Acetonitrile 95:5 to 5:95 (v/v) in 90 min temperature: not reported flow-rate: 0.8 mL/min | ESI Negative Capillary voltage: 3 Kv Dry temperature: 360 °C | Full Scan SIR quantification | Group B: Soyasaponin I Soyasapogenol E and B |
| Waters 2690 Alliance HPLC system coupled with a Micromass Mass spectrometer [22] | Supelcosil LC-18-DB column A: 0.2% formic acid in water (v/v) B: 0.2% formic acid in Methanol (v/v) temperature: not reported flow-rate: 1mL/min | ESI Negative Capillary voltage: 3.7 Kv Dry temperature: 200 °C | Full Scan SIR quantification | Group A: Aa, Ab, Ac, Ae, Af, Ag and Ah Group B: Ba, Bb, Bc, Bb’, Bc’, Bd Group E: Be |
| Dynamax Model SD-200 with Hewlett-Packard HP5898 B quadrupole Mass Spectrometer [42] | SupLC-18 microbore column A: 30% Acetonitrile in water (v/v) B: 100% Acetonitrile temperature: not reported flow-rate: 0.1 mL/min | ESI Positive and Negative Capillary voltage: not reported Dry temperature: 150 °C | Full Scan | Group B: I, II and V DDMP βg Group A: Acetylsoysaponin A4 |
| FAB MS System | FAB MS Condition | FAB MS Mode | Detected Soyasaponins | |
| JEOL JMS SX 102/102 high-resolution double-focusing four-sector tandem mass spectrometer (FAB/MS) [42] | Full accelerating voltage of 10 keV Resolving power 3 x 103 Xenon was used for providing fast atoms 20 mA discharge current Magnet scan rate: 10s per decade | Positive and Negative Full Scan MS/MS Detection | Group B: I, II and V DDMP βg Group A: Acetyl soyasaponin A4 | |
Hydrolysis
| Soyasaponin | Mass | Ion Fragments, m/z | |
|---|---|---|---|
| [M+H]+ | Others | ||
| I | 942 | 943.1 | 1045.9, 945.2, 944.1, 913.1, 531.7, 142.7 |
| II | 912 | 913.1 | 1045.0, 1014.2, 944.1, 914.1, 532.0, 516.7, 142.6 |
| III | 796 | 797.2 | 1015.0, 914.0, 913.1, 799.2, 142.6 |
| IV | 766 | 767.1 | 913.1, 769.1, 536.2, 464.4, 142.6 |
| V | 958 | 959.2 | 1029.2, 961.2, 960.2, 519.2, 142.7 |
| βg | 1068 | 1069.2 | 911.0, 594.6, 142.8 |
| βa | 1038 | 1039.0 | 795.2, 579.8, 142.6 |
| γg | 922 | 923.2 | 924.2, 925.2, 501.6, 142.6 |
| γa | 892 | 893.2 | 894.1, 923.2, 924.1, 1012.8, 566.6, 527.2, 142.5 |
| Be | 940 | 941.1 | 942.1, 531, 142.8 |
Measured bioactivities of soyasaponins in cell culture
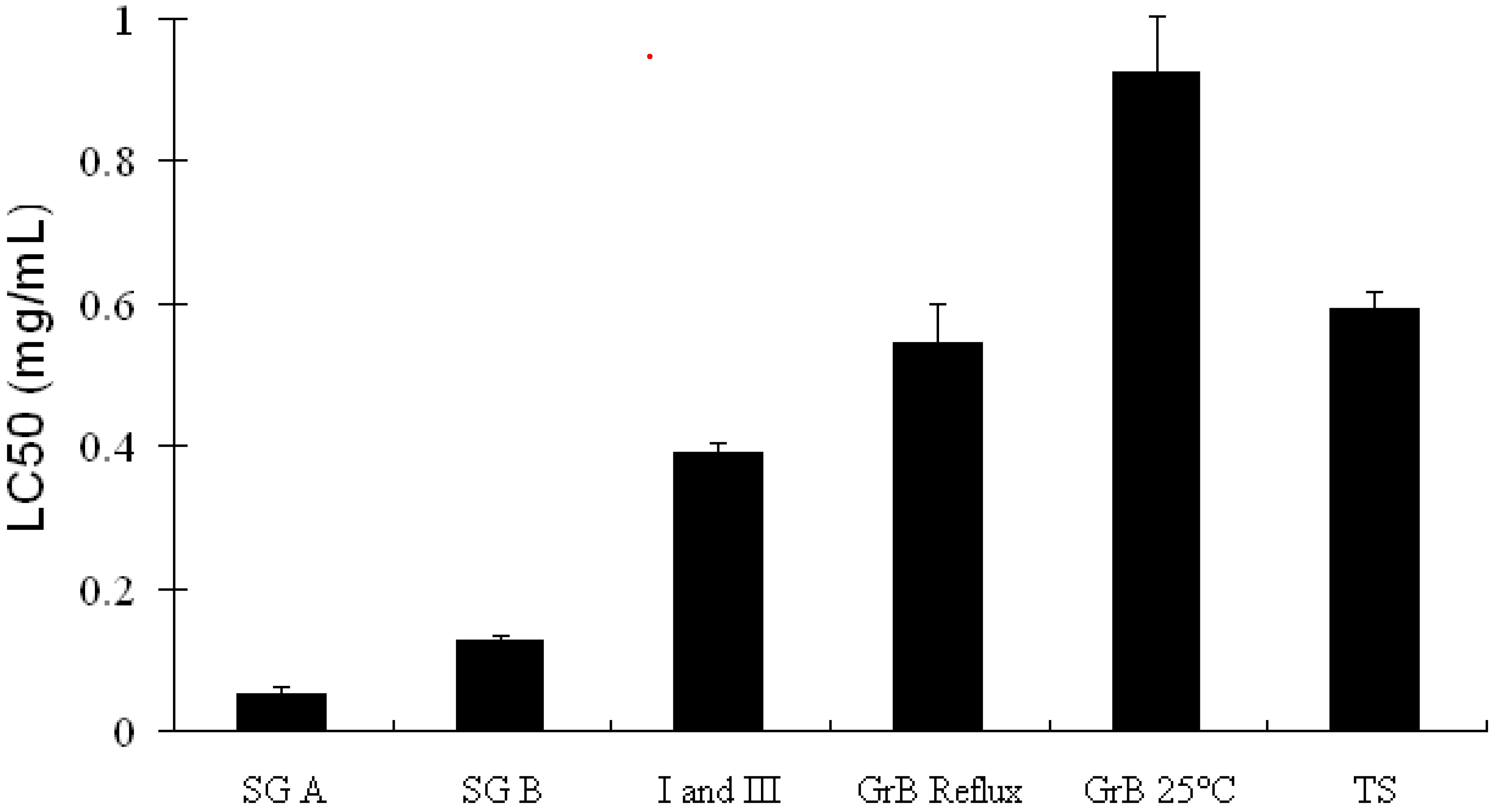
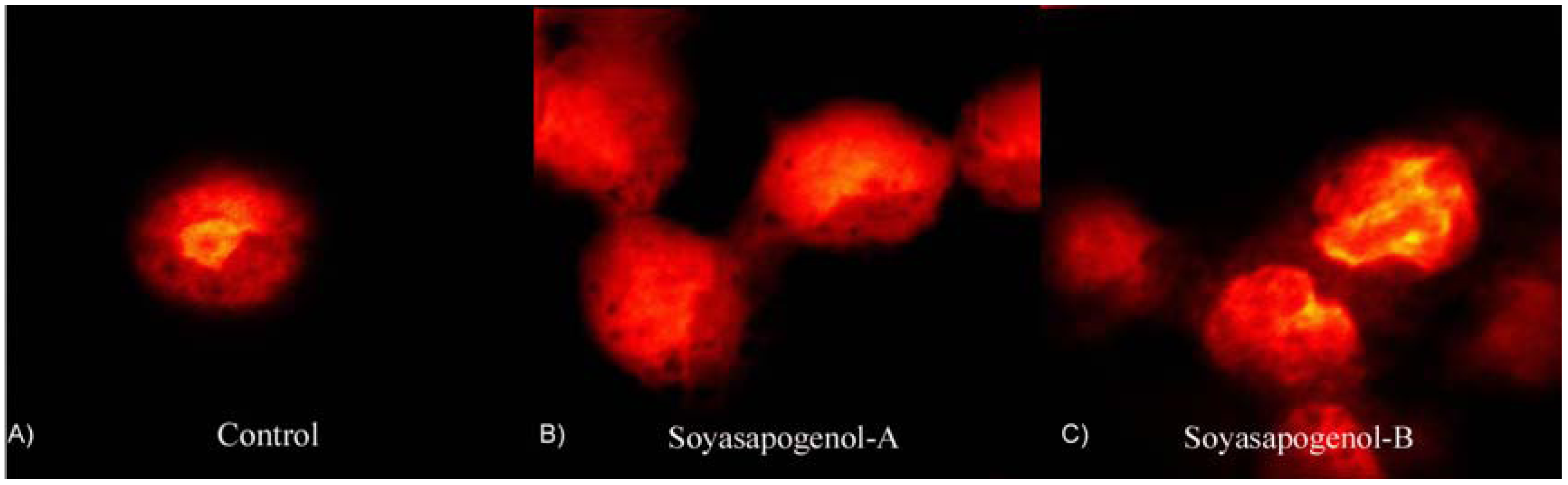
Soyasaponins and apoptosis
Sialytransferase activity
Conclusions
Acknowledgements
References and Notes
- Daveby, Y.D.; Aman, P.; Betz, J.M.; Musser, S.M. Effect of Storage and Extraction on Ratio of Soyasaponin I to 2,3-Dihydro-2,5-dihydroxy-6- methyl-4-pyrone-Conjugated Soyasaponin I in Dehulled Peas (Pisum sativum L). J. Sci. Food Agric. 1998, 141–146. [Google Scholar]
- Ruiz, R.G.; Price, K.R.; Arthur, A.E.; Rose, M.E.; Rhodes, M.J.C.; Fenwick, R.G. Effect of soaking and cooking on the saponin content and composition of chickpeas (Cicer arietinum) and lentils (Lens culinaris). J. Agric. Food Chem. 1996, 44, 1526–1530. [Google Scholar] [CrossRef]
- Yoshiki, Y.; Kudou, S.; Okubo, K. Relationship between chemical structures and biological activities of triterpenoid saponins from soybean. Biosci. Biotechnol. Biochem. 1998, 62, 2291–2299. [Google Scholar] [CrossRef]
- Kitagawa, I.; Saito, M.; Taniyama, T.; Yoshikawa, M. Saponin and Sapogenol. XXXVIII. Structure of Soyasaponin A_2,a Bisdesmoside of Soyasapogenol A, from soybean, the seeds of glycine max MERRILL. Chem. Pharm. Bull. 1985, 33, 598–608. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Harada, K.; Okubo, K. Composition and structure of group-B saponin in soybean seed. Agric. Biol. Chem. 1991, 55, 911–917. [Google Scholar] [CrossRef]
- Kudou, S.; Tonomura, M.; Tsukamoto, C.; Shimoyamada, M.; Uchida, T.; Okubo, K. Isolation and structural elucidation of the major genuine soybean saponin. Biosci. Biotechnol. Biochem. 1992, 56, 142–143. [Google Scholar] [CrossRef]
- Kudou, S.; Tonomura, M.; Tsukamoto, C.; Uchida, T.; Sakabe, T.; Tamura, N.; Okubo, K. Isolation and structural elucidation of DDMP-conjugated soyasaponins as geniune saponins from soybean seeds. Biosci. Biotechnol. Biochem. 1993, 57, 546–550. [Google Scholar] [CrossRef]
- Kitagawa, I.; Yoshikawa, M.; Wang, H.; Saito, M.; Tosirisuk, V.; Fujiwara, T.; Tomita, K. Revised structures of soyasapogenols A, B, and E, oleanene-sapogenols from soybean. Structures of soyasaponins I, II, and III. Chem. Pharm. Bull. 1982, 30, 2294–2297. [Google Scholar] [CrossRef]
- Gurfinkel, D.M.; Rao, A.V. Soyasaponins: The Relationship Between Chemical Structure and Colon Anticarcinogenic Activity. Nutr. Cancer 2003, 47, 24–33. [Google Scholar] [CrossRef]
- MacDonald, R.S.; Guo, J.Y.; Copeland, J.; Browning, J.D.; Sleper, D.; Rottinghaus, G.E.; Berhow, M.A. Environmental influences on isoflavones and saponins in soybeans and their role in colon cancer; Amer Inst Nutrition: Washington, DC, 2004; pp. 1239–1242. [Google Scholar]
- Tsukamoto, C.; Kikuchi, A.; Kudou, S.; Harada, K.; Kitamura, K.; Okubo, K. Group A acetyl saponin-deficient mutant from the wild soybean. Phytochemistry 1992, 31, 4139–4142. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Jackson, C.J.C.; Poysa, V.; Di Berardo, C.; Bewley, J.D.; Jenkinson, J. Soyasapogenol A and B distribution in soybean (Glycine max L. Merr.) in relation to seed physiology, genetic variability, and growing location. J. Agric. Food Chem. 2003, 51, 5888–5894. [Google Scholar] [CrossRef]
- Ireland, P.A.; Dziedzic, S.Z. Effect of hydrolysis on sapogenin release in soya. J. Agric. Food Chem. 1986, 34, 1037–1041. [Google Scholar] [CrossRef]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef]
- Zhang, W.; Popovich, D.G. Soyasaponins I and III derived from soy flour induce apoptoisis in Hep-G2 cells. Pharm. Biol. 2009, 47, 34. [Google Scholar] [CrossRef]
- Zhang, W.; Popovich, D.G. Effect of Soyasapogenol A and Soyasapogenol B Concentrated Extracts on Hep-G2 Cell Proliferation and Apoptosis. J. Agric. Food Chem. 2008, 56, 2603–2608. [Google Scholar] [CrossRef]
- Sumiki, Y. Studies on the saponin of soy-bean. Bull. Agric. Chem. Soc. Japan 1929, 5, 27–32. [Google Scholar] [CrossRef]
- Hu, J.; Lee, S.O.; Hendrich, S.; Murphy, P.A. Quantification of the group B soyasaponins by high-performance liquid chromatography. J. Agric. Food Chem. 2002, 50, 2587–2594. [Google Scholar] [CrossRef]
- Zhang, W.; Teng, S.P.; Popovich, D.G. Generation of Group B Soyasaponins I and III by Hydrolysis. J. Agric. Food Chem. 2009, 57, 3620–3625. [Google Scholar] [CrossRef]
- Decroos, K.; Vincken, J.P.; van Koningsveld, G.A.; Gruppen, H.; Verstraete, W. Preparative chromatographic purification and surfactant properties of individual soyasaponins from soy hypocotyls. Food Chem 2007, 101, 324–333. [Google Scholar] [CrossRef]
- Tava, A.; Mella, M.; Bialy, Z.; Jurzysta, M. Stability of saponins in alcoholic solutions: Ester formation as artifacts. J. Agric. Food Chem. 2003, 51, 1797–1800. [Google Scholar] [CrossRef]
- Gu, L.W.; Tao, G.J.; Gu, W.Y.; Prior, R.L. Determination of soyasaponins in soy with LC-MS following structural unification by partial alkaline degradation. J. Agric. Food Chem. 2002, 50, 6951–6959. [Google Scholar] [CrossRef]
- Gurfinkel, D.M.; Reynolds, W.F.; Rao, A.V. The isolation of soyasaponins by fractional precipitation, solid phase extraction, and low pressure liquid chromatography. Int. J. Food Sci. Nutr. 2005, 56, 501–519. [Google Scholar] [CrossRef]
- Popovich, D.G.; Kitts, D.D. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry 2004, 65, 337–344. [Google Scholar] [CrossRef]
- Zhang, W.; Yeo, M.C.; Tang, F.Y.; Popovich, D.G. Bioactive responses of Hep-G2 cells to Soyasaponin extracts differs with respect to extraction conditions. Food Chem. Toxicol. 2009, in press. [Google Scholar]
- Gurfinkel, D.M.; Rao, A.V. Determination of saponins in legumes by direct densitometry. J. Agric. Food Chem. 2002, 50, 426–430. [Google Scholar] [CrossRef]
- Hubert, J.; Berger, M.; Dayde, J. Use of a simplified HPLC-UV analysis for soyasaponin B determination: Study of saponin and isoflavone variability in soybean cultivars and soy-based health food products. J. Agric. Food Chem. 2005, 53, 3923–3930. [Google Scholar] [CrossRef]
- Ganzera, M.; Stuppner, H.; Khan, I.A. Simultaneous determination of saponins and isoflavones in soybean (Glycine max L.) by reversed-phase liquid chromatography with evaporative light-scattering and ultraviolet detection. J. AOAC Int. 2004, 87, 1189–1194. [Google Scholar]
- Decroos, K.; Vincken, J.P.; Heng, L.; Bakker, R.; Gruppen, H.; Verstraete, W. Simultaneous quantification of differently glycosylated, acetylated, and 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one-conjugated soyasaponins using reversed-phase high-performance liquid chromatography with evaporative light scattering detectio. J. Chromatogr. A 2005, 1072, 185–193. [Google Scholar]
- Yang, Y.; Jin, M.; Huang, M.; Su, B.; Ren, Q. Ultrasound-assisted extraction of soyasaponins from hypocotyls, and analysis by LC-ESI-MS. Chromatographia 2007, 65, 555–560. [Google Scholar]
- Berhow, M.A.; Kong, S.B.; Vermillion, K.E.; Duval, S.M. Complete quantification of group A and group B soyasaponins in soybeans. J. Agric. Food Chem. 2006, 54, 2035–2044. [Google Scholar] [CrossRef]
- Jin, M.; Yang, Y.; Su, B.; Ren, Q. Rapid quantification and characterization of soyasaponins by high-performance liquid chromatography coupled with electrospray mass spectrometry. J. Chromatogr. A 2006, 1108, 31–37. [Google Scholar]
- Ireland, P.A.; Dziedzic, S.Z. Analysis of soybean sapogenins by high-performance liquid chromatography. J. Chromatogr. A 1985, 325, 275–281. [Google Scholar]
- Berhow, M.A.; Cantrell, C.L.; Duval, S.M.; Dobbins, T.A.; Maynes, J.; Vaughn, S.F. Analysis and quantitative determination of group B saponins in processed soybean products. Phytochem. Anal. 2002, 13, 343–348. [Google Scholar] [CrossRef]
- Hubert, J.; Berger, M.; Dayde, J. Validation of a high-performance liquid chromatography-ultraviolet method to quantify soy sapogenols A and B in soy germs from different cultivars and in soy isoflavone-enriched supplements. J. Food Sci. 2005, 70, C471–C477. [Google Scholar] [CrossRef]
- Popovich, D.G.; Kitts, D.D. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry 2004, 65, 337–344. [Google Scholar]
- Hu, J.; Zheng, Y.L.; Hyde, W.; Hendrich, S.; Murphy, P.A. Human fecal metabolism of soyasaponin I. J. Agric. Food Chem. 2004, 52, 2689–2696. [Google Scholar] [CrossRef]
- Heftmann, E.; Luudin, R.E.; Haddon, W.F.; Peri, I.; Mor, U.; Bondi, A. High-pressure liquid chromatography, nuclear magnetic resonance and mass spectra of biosynthetic soyasapogenols. J. Nat. Prod. 1979, 42, 410–416. [Google Scholar] [CrossRef]
- Jin, M.; Yang, Y.; Su, B.; Ren, Q. Determination of soyasaponins Ba and Bb in human serum by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 846, 169–175. [Google Scholar] [CrossRef]
- Dalluge, J.J.; Eliason, E.; Frazer, S. Simultaneous identification of soyasaponins and isoflavones and quantification of soyasaponin Bb in soy products, using liquid chromatography/electrospray ionization-mass spectrometry. J. Agric. Food Chem. 2003, 51, 3520–3524. [Google Scholar] [CrossRef]
- Huhman, D.V.; Berhow, M.A.; Sumner, L.W. Quantification of saponins in aerial and subterranean tissues of Medicago truncatula. J. Agric. Food Chem. 2005, 53, 1914–1920. [Google Scholar] [CrossRef]
- Lee, M.R.; Chen, C.M.; Hwang, B.H.; Hsu, L.M. Analysis of saponins from black bean by electrospray ionization and fast atom bombardment tandem mass spectrometry. J. Mass Spectrom. 1999, 34, 804–812. [Google Scholar] [CrossRef]
- Kitts, D.D.; Popovich, D.G. Ginseng. In Performance Functional Foods; Watson, D., Ed.; Woodhead Publishing LTD.: New York, NY, USA, 2003; pp. 78–88. [Google Scholar]
- Xiao, J.X.; Huang, G.Q.; Zhang, S.H. Soyasaponins inhibit the proliferation of Hela cells by inducing apoptosis. Exp. Toxicol. Pathol. 2007, 59, 35–42. [Google Scholar] [CrossRef]
- Ellington, A.A.; Berhow, M.; Singletary, K.W. Induction of macroautophagy in human colon cancer cells by soybean B-group triterpenoid saponins. Carcinogenesis 2005, 26, 159–167. [Google Scholar] [CrossRef]
- Milgate, J.; Roberts, D.C.K. The nutritional and biological significance of saponins. Nutr. Res. 1995, 15, 1223–1249. [Google Scholar] [CrossRef]
- Popovich, D.G.; Kitts, D.D. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch. Biochem. Biophys. 2002, 406, 1–8. [Google Scholar] [CrossRef]
- Ellington, A.A.; Berhow, M.A.; Singletary, K.W. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis 2006, 27, 298–306. [Google Scholar] [CrossRef]
- Kanduc, D.; Mittelman, A.; Serpico, R.; Sinigaglia, E.; Sinha, A.A.; Natale, C.; Santacroce, R.; Di Corcia, M.G.; Lucchese, A.; Dini, L.; Pani, P.; Santacroce, S.; Simone, S.; Bucci, R.; Farber, E. Cell death: apoptosis versus necrosis (review). Int. J. Oncol. 2002, 21, 165–170. [Google Scholar]
- Koratkar, R.; Rao, A.V. Effect of soya bean saponins on azoxymethane-induced preneoplastic lesions in the colon of mice. Nutr. Cancer 1997, 27, 206–209. [Google Scholar] [CrossRef]
- Sung, M.K.; Kendall, C.W.C.; Koo, M.M.; Rao, A.V. Effect of soybean saponins and gypsophilla saponin on growth and viability of colon-carcinoma cells in culture. Nutr. Cancer 1995, 23, 259–270. [Google Scholar] [CrossRef]
- Sung, M.K.; Kendall, C.W.C.; Rao, A.V. Effect of soybean saponins and gypsophila saponin on morphology of colon-carcinoma cells in culture. Food Chem. Toxicol. 1995, 33, 357–366. [Google Scholar] [CrossRef]
- Yoshikoshi, M.; Yoshiki, Y.; Okubo, K.; Seto, J.; Sasaki, Y. Prevention of hydrogen peroxide damage by soybean saponins to mouse fibroblasts. Planta Med. 1996, 62, 252–255. [Google Scholar] [CrossRef]
- Oh, Y.J.; Sung, M.K. Soybean saponins inhibit cell proliferation by suppressing PKC activation and induce differentiation of HT-29 human colon adenocarcinoma cells. Nutr. Cancer 2001, 39, 132–138. [Google Scholar] [CrossRef]
- Popovich, D.G.; Kitts, D.D. Ginsenosides 20(S)-protopanaxadiol and Rh2 reduce cell proliferation and increase sub-G1 cells in two cultured intestinal cell lines, Int-407 and Caco-2. Can. J. Physiol. Pharmacol. 2004, 82, 183–190. [Google Scholar] [CrossRef]
- Rowlands, J.C.; Berhow, M.A.; Badger, T.M. Estrogenic and antiproliferative properties of soy sapogenols in human breast cancer cells in vitro. Food Chem. Toxicol. 2002, 40, 1767–1774. [Google Scholar] [CrossRef]
- Yanamandra, N.; Berhow, M.A.; Konduri, S.; Dinh, D.H.; Olivero, W.C.; Nicolson, G.L.; Rao, J.S. Triterpenoids from Glycine max decrease invasiveness and induce caspase-mediated cell death in human SNB19 glioma cells. Clin. Exp. Metastasis 2003, 20, 375–383. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Another way to die: autophagic programmed cell death. Cell Death. Differ. 2005, 12 (Suppl 2), 1528–1534. [Google Scholar] [CrossRef]
- Gessner, P.; Riedl, S.; Quentmaier, A.; Kemmner, W. Enhanced activity of CMP-NEUAC-GAL-beta-1-4GLCNAC-alpha-2,6-sialytransferase in metabstasizing human colorectal tumor-tissue and serum of tumor patients. Cancer Lett. 1993, 75, 143–149. [Google Scholar] [CrossRef]
- Majuri, M.L.; Niemela, R.; Tiisala, S.; Renkonen, O.; Renkonen, R. Expression and function of alpha-2,3-sialyl-transferases and alpha-1,3/1,4-fucosyl-transferaes in colon adenocarcinoma cell-line - role in synthesis of E-selectin counter-receptors. Int. J. Cancer 1995, 63, 551–559. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hus, C.C.; Chen, S.T.; Tsai, Y.C. Soyasaponin I, a potent and specific sialyltransferase inhibitor. Biochem. Biophys. Res. Commun. 2001, 284, 466–469. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, T.W.; Chang, W.W.; Wu, C.Y.; Lo, W.H.; Wang, P.H.; Tsai, Y.C. Soyasaponin-I-modified invasive behavior of cancer by changing cell surface sialic acids. Gynecol. Oncol. 2005, 96, 415–422. [Google Scholar] [CrossRef]
- Chang, W.W.; Yu, C.Y.; Lin, T.W.; Wang, P.H.; Tsai, Y.C. Soyasaponin I decreases the expression of alpha 2,3-linked sialic acid on the cell surface and suppresses the metastatic potential of B16F10 melanoma cells. Biochem. Biophys. Res. Commun. 2006, 341, 614–619. [Google Scholar] [CrossRef]
- Sample availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, W.; Popovich, D.G. Chemical and Biological Characterization of Oleanane Triterpenoids from Soy. Molecules 2009, 14, 2959-2975. https://doi.org/10.3390/molecules14082959
Zhang W, Popovich DG. Chemical and Biological Characterization of Oleanane Triterpenoids from Soy. Molecules. 2009; 14(8):2959-2975. https://doi.org/10.3390/molecules14082959
Chicago/Turabian StyleZhang, Wei, and David G. Popovich. 2009. "Chemical and Biological Characterization of Oleanane Triterpenoids from Soy" Molecules 14, no. 8: 2959-2975. https://doi.org/10.3390/molecules14082959




