Vibrational Spectra Study of the Interactions Between Keggin Heteropolyanions and Amino Acids
Abstract
:1. Introduction
2. Results and Discussion
| Entry | HPA and HPA with amino acid | Vibrational frequencies |
|---|---|---|
| 1 | H3[PW12O40] | 1080(s), 990(sh), 982(s), 890(s), 810(vs), 597(w), 527(m) |
| 2 | Na3[PW12O40] | 1081(s), 995(sh), 982(s), 922(m), 900(m) , 805(vs), 592(w), 522(sh) |
| 3 | H4[SiW12O40] | 1020(w), 981(s), 928(vs), 880(m), 785(vs), 552(sh), 540(m) |
| 4 | Na2H[PMo12O40] | 1068(s), 978(sh), 962(vs), 869(s), 785(vs), 593(w) |
| 5 | H3[PMo12O40] | 1064(s), 975(sh), 963(vs), 870(s), 810(sh), 785(vs), 593(w) |
| 6 | H4[SiMo12O40] | 995(sh), 957(s), 904(vs), 855(m),770(vs), 535(m), 442(w) |
| 7 | [leucine]xHy [PMo12O40] | 1061.8(s), 973.4(sh), 961.4(vs), 867(w), 891(s), 810(sh), 794(vs), 739.1(sh), 677.3(sh), 668.7(s), 633.5(sh) |
| 8 | [phenylalanine]xHy[PMo12O40] | 1062.7(s), 975(sh), 966.5(vs), 892(sh), 871.3(w), 889(s), 810(sh), 795(vs), 739.1(sh), 605.7(sh), 669.6(m) |
| 9 | [glycine]xHy[PMo12O40] | 977.7(w), 975(sh), 955.4(w), 909(vw), 925.3(w), 835.2(sh), 732(w), 668.7(s) |
| 10 | [phenylalanine]x Nay[PMo12O40] | 1061.8(vs), 975(sh), 967(vs), 891(s), 871.4(w), 800.6(vs), 741.7(vw), 668.2(s), 608.5(sh) |
| 11 | [valine]xHy [PMo12O40] | 1062.7(s), 973.4(sh), 963.1(vs), 886.7(m), 867(m), 810(sh), 795.9(sh), 790.3(vs), 740.8(sh), 667.8(s) |
| 12 | [leucine]xNay[PMo12O40] | 1064.4(vs), 970(vs), 959.7(s), 896.1(s), 857.5(w), 791.9(vs), 740(sh), 673.8(sh), 668.7(s), 636.1(w) |
| 13 | [glycine]xNay[PMo12O40] | 1116.8(vw), 976.9(w), 976.9(w), 954.5(s), 922.7(s), 908.2(w), 836.9(sh), 733.1(sh), 675.6(sh), 667.8(s), 610.3(sh) |
| 14 | [valine]xHy[PW12O40] | 1080.7(vs), 990(sh), 982.8(vs), 995(sh), 893.6(vs), 811.2(vs), 738.2(w) 668.7(s) |
| 15 | [phenylalanine]xHy[PW12O40] | 1061.3(w), 976.9(s), 990(sh), 924.5(s) 880(sh), 794(s) |
| 16 | [leucine]xHy[SiW12O40] | 1133.1(w), 975.1(vs), 1015.5(s), 975.1(vs), 985.1(sh), 922.7(vs), 890.1(w), 794(b), 667.8(s), 635.2(w) |
| 17 | [valine]xHy [SiW12O40] | 1016.3(s), 971.7(vs), 981(sh), 924.5(vs). 888.4(w), 867(w), 794(vs), 679(sh), 667.8(s), 635.2(w) |
| 18 | [glycine]xHy[SiW12O40] | 1014.6(s), 972.5(vs), 989(sh), 925(vs), 935.3(sh), 889.3(w), 788.9(vs), 667.8(s) |
| 19 | [glycine]xHy[SiMo12O40] | 1073.7(vw), 976.4(m), 896.2(vw), 837.3(w), 732.2(w), 678.5(sh), 669.1(s) |
| 20 | [valine]xHy[SiMo12O40] | 1079(vw), 976.9(w), 897.9(w), 834.4(w), 733.1(w),668.7(s), 615.5(sh) |
| 21 | [leucine]xHy[SiMo12O40] | 1063.5(vw), 976.9(w), 904.7(w), 790.6(vw), 737.4(w), 668.7(s), 614.6(sh) |
| 22 | [phenylalanine]xHy[SiW12O40] | 1071.2(sh), 960.9(s), 920.9(s), 891(sh), 880.9(w), 747.8(w), 668(s) |
| 23 | [glycine] xHy[PW12O40] | 976.9(w), 900(w), 838.6(vw), 830.1(vw), 788(w), 733.9(m), 668.7(s) |
| 24 | [leucine]xHy[PW12O40] | 1081.3(vs), 979.4(s), 896.5(s), 807(w), 740.8(w), 678.8(sh), 668.6(s) |
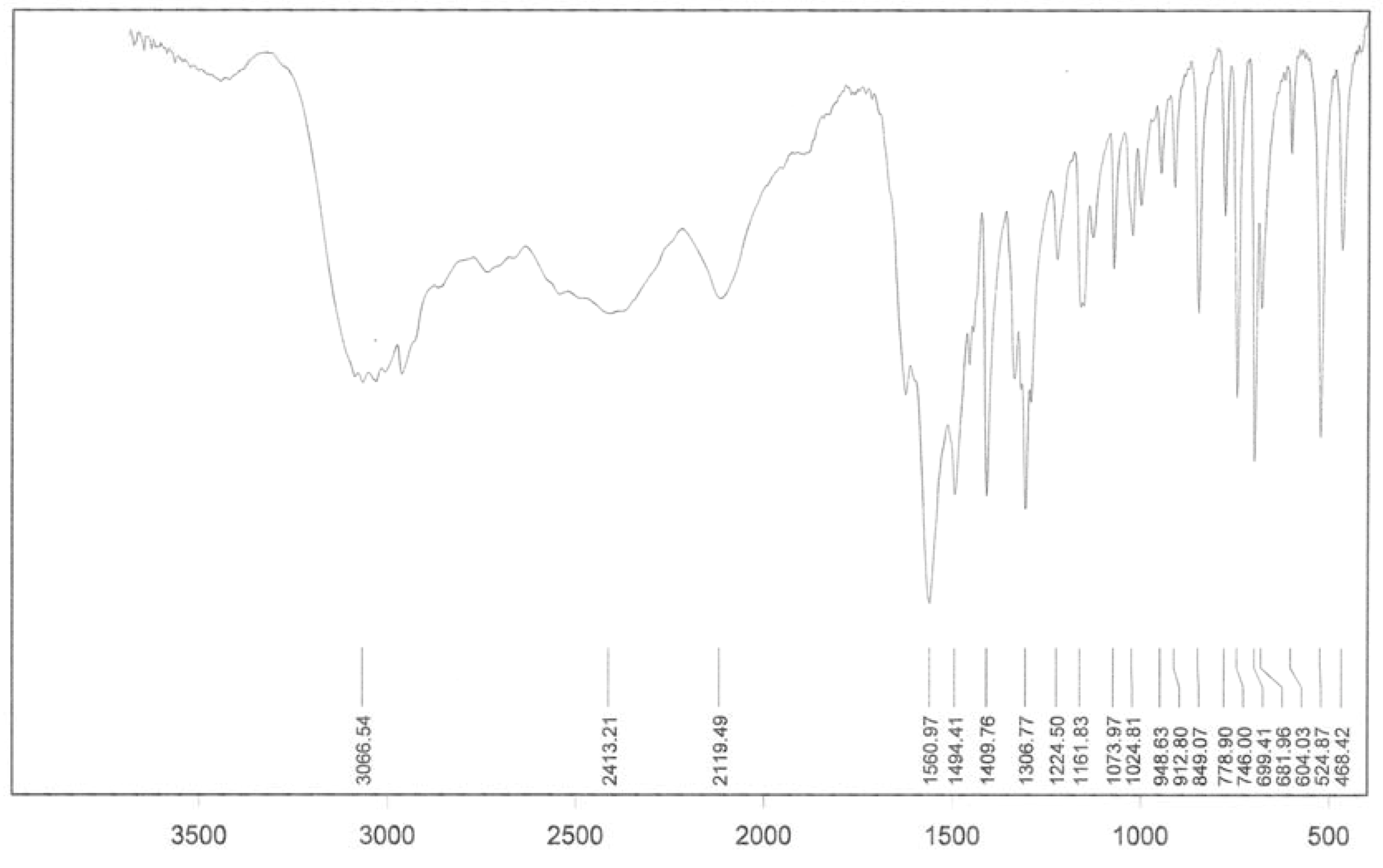
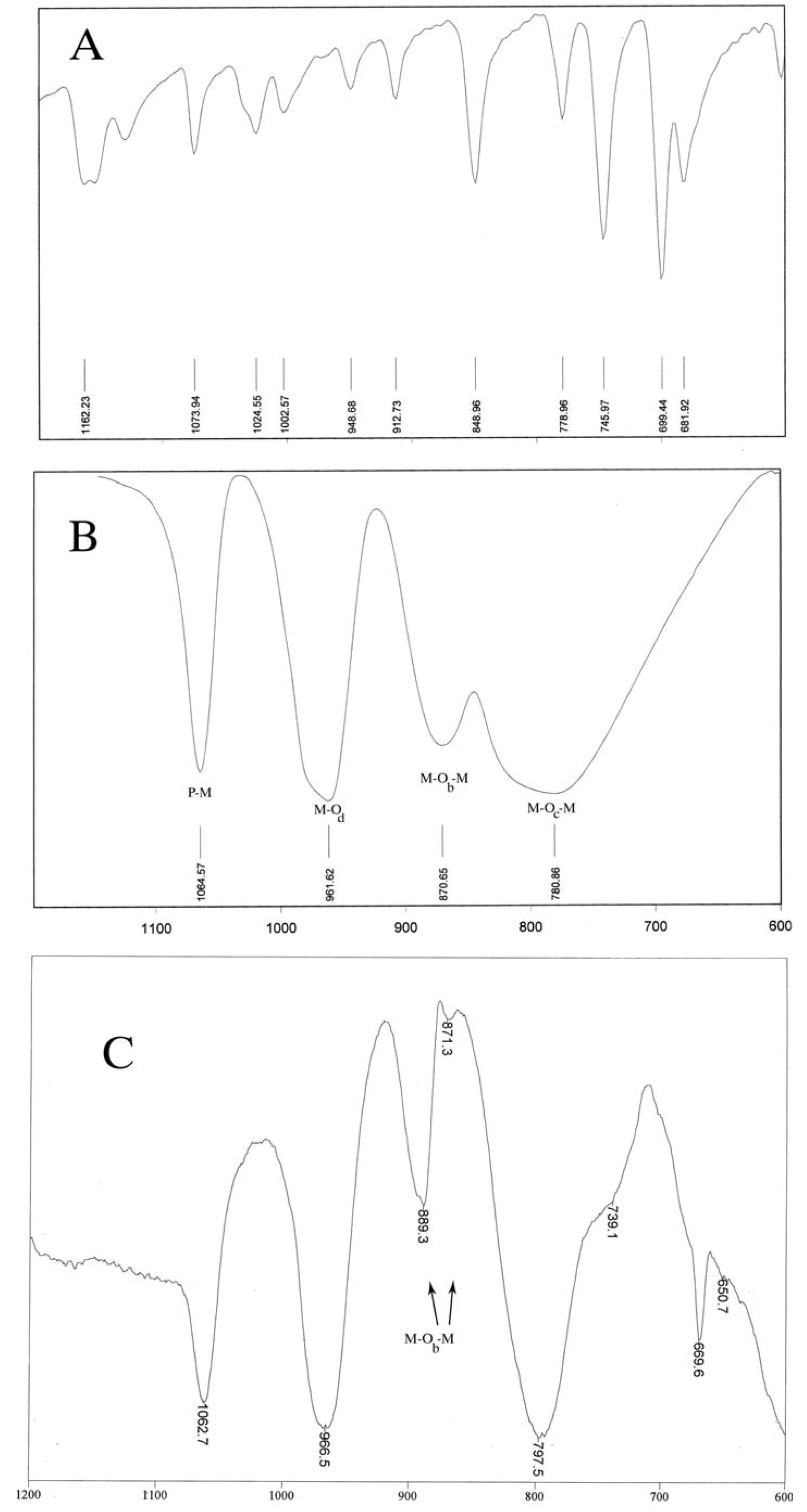
3. Experimental
3.1. General
3.2. General procedure for the preparation of amino acid-heteropolyanion adducts
- Sample Availability: Samples of the compounds in Table 1 are available from the authors.
References
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M.; Gharib, A. Preyssler catalyst, [NaP5W30 O110]14-: A green, efficient and reusable catalyst for esterification of salicylic acid with aliphatic and benzylic alcohols. Appl. Catal. A Gen. 2006, 302, 42–47. [Google Scholar]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Tavakoli, N. N-oxidation of pyridine carboxylic acids using hydrogen peroxide catalyzed by a green heteropolyacid catalyst: Preyssler's anion, [NaP5W30O110]14-. J. Mol. Catal. A Chem. 2006, 252, 219–225. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Gharib, A.; Jahangir, M. A catalytic method for synthesis ofγ- butyrolactone, ε-caprolactone and 2-cumaranone in the presence of Preyssler's anion, [NaP5W30O110 ]14-, as a green and reusable catalyst. J. Mol. Catal. A Chem. 2006, 252, 90–95. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Akbarpour, M. Catalytic performance of Preyssler heteropolyacid as a green and recyclable catalyst in oxidation of primary aromatic amines. J. Mol. Catal. A Chem. 2006, 255, 193–198. [Google Scholar]
- Bamoharram, F.F.; Heravi, M.M.; Heravi, M.M.; Meraji, M. Synthesis of Silver Nanoparticles in the Presence of A Green Heteropolyacid, H14[NaP5W30O110 ], and Their Catalytic Activity for Photodegradation of Methylene Blue and Methyl Orange”. Int. J. Green Nanotech. 2009, in press. [Google Scholar]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Toosi, M. Synthesis and characterization of silica-supported Preyssler nanoparticles and its catalytic activity for photodegradation of methyl orange. Green Chem. Lett. Rev. 2009, 2, 35–41. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M.; Gharib, A. Effective direct esterification of butanol by eco-friendly Preyssler catalyst, [NaP5W30O110]14−. J. Mol. Catal. A Chem. 2007, 271, 126–130. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Roshani, M.; Alizadeh, M.H.; Razavi, H.; Moghayadi, M. Novel oxidation of aromatic aldehydes catalyzed by Preyssler’s anion, [NaP5W30O110]14-". J. Braz. Chem. Soc. 2006, 17, 505–509. [Google Scholar] [CrossRef]
- Lomakina, S.V.; Shatova, T.S.; Kazansky, L.P. Heteropoly anions as corrosion inhibitors for aluminium in high temperature water. Corros. Sci. 1994, 36, 1645–1655. [Google Scholar] [CrossRef]
- Tasumisago, M.; Honjo, H.; Sakai, Y.; Minami, T. Proton-conducting silica-gel films doped with a variety of electrolytes. Solid State Ionics 1994, 74, 105–108. [Google Scholar] [CrossRef]
- Sheu, H.C.; Shih, J.S. Organic ammonium ion selective electrodes based on 18-crown-6-phosphotungstic acid precipitates. Anal. Chim. Acta 1996, 324, 125–134. [Google Scholar]
- Giordano, N.; Staiti, P.; Arico, A.S.; Passalacqua, E.; Abate, L.; Hocevar, S. Analysis of the chemical cross-over in a phosphotungstic acid electrolyte based fuel cell. Electrochim. Acta 1997, 42, 1645–1652. [Google Scholar] [CrossRef]
- Rhule, J.T.; Hill, C.L.; Judd, D.A. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar]
- Kozhevnikov, I.V. Catalysis by Polyoxometalates; Wiley & Sons: Chichester, UK, 2002. [Google Scholar]
- Crans, D.C. Enzyme interactions with labile oxovanadates and other oxometalates. Comm. Inorg.Chem. 1994, 16, 35–76. [Google Scholar]
- Yamamoto, N.; Schols, D.; De Clercq, E.; Debyser, Z.; Pauwels, R.; Balzarini, J.; Nakashima, H.; Baba, M.; Hosoya, M.; Snoeck, R.; Neyts, J.; Andrei, G.; Murrer, B.A.; Theobald, B.; Bossard, G.; Henson, G.; Abrams, M.; Picker, D. Mechanism of anti-human immunodeficiency virus action of polyoxometalates, a class of broad-spectrum antiviral agents. Mol. Pharmacol. 1992, 42, 1109–1117. [Google Scholar]
- Inouye, Y.; Tokutake, Y.; Yoshida, T.; Seto, Y.; Hujita, H.; Dan, K.; Yamamoto, A.; Nishiya, S.; Yamase, T.; Nakamura, S. In vitro antiviral activity of polyoxomolybdates. Mechanism of inhibitory effect of PM-104(NH4)12H2(Eu4(MoO4)(H2O)16(Mo7O24)4).13H2O on human immunodeficiency virus type 1. Antiviral Res. 1993, 20, 317–331. [Google Scholar] [CrossRef]
- Liu, J.; Peng, J.; Wang, E.; Bi, L.; Guo, S. A novel amino acid salt of 18-molybdodiphosphate: synthesis and structural characterization of (Lys)2H6[P2Mo18O62]·16H2O. J. Mol. Struct. 2000, 525, 71–77. [Google Scholar] [CrossRef]
- Crans, D.C.; Mahroof-Tahir, M.; Anderson, O.P.; Miller, M.M. X-ray Structure of (NH4)6(Gly-Gly)2V10O28.cntdot.4H2O: Model Studies for Polyoxometalate-Protein Interactions. Inorg. Chem. 1994, 33, 5586–5590. [Google Scholar] [CrossRef]
- Attanasio, D.; Bonamico, M.; Fares, V.; Imperatori, P.; Suber, L. Weak charge-transfer polyoxoanion salts: the reaction of quinolin-8-ol (Hquin) with phosphotungstic acid and the crystal and molecular structure of [H2quin]3[PW12O40]·4EtOH·2H2O. J. Chem. Soc. Dalton Trans. 1990, 3221–3228. [Google Scholar]
- You, W.; Wang, E.; He, Q.; Xu, L.; Xing, Y.; Jia, H. Synthesis and crystal structure of a new supermolecular compound: [C12H24O6][H3PMo12O40]·22H2O (C12H24O6=18-crown-6). J. Mol. Struct. 2000, 524, 133–139. [Google Scholar] [CrossRef]
- Fu, X.K.; Chen, J.R.; Li, L.Q.; Wang, Q.; Sui, Y. Organophosphonotungstic HPA of Keggin Type with Sulfo, Taurine and Glycine Substituted Ethylphosphonic Acids as the Coordinate Center. Chin. Chem. Lett. 2003, 14, 515–518. [Google Scholar]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207–216. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Thouvenot, R.; Dabbabi, M. Etude de la structure des niobotungstates NbnW6—nO−2—n19 au moyen des spectres de vibration. Spectrochim. Acta 1977, 33, 143–153. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Thouvenot, R.; Franck, R. Spectres i.r. et Raman d'hétéropolyanions α- XM12O40n− de structure de type Keggin (X = BIII, SiIV, GeIV, PV, AsV et M = WVI et MoVI). Spectrochim. Acta 1976, 32, 587–597. [Google Scholar] [CrossRef]
- Peng, J.; Wang, E.; Zhou, Y.; Xing, Y.; Jia, H.; Lin, Y.; Shen, Y. Synthesis, electrical properties and crystal structure of a new radical-ion salt based on π-extended bis(TTF) and a Keggin heteropolymolybdate. J. Chem. Soc. Dalton Trans. 1998, 3865–3870. [Google Scholar]
- Lihua, B.; Qizhuang, H.; Qiong, J.; Enbo, W. Synthesis, properties and crystal structure of (Gly)2H4SiW12O40·5.5H2O. J. Mol. Struct. 2001, 597, 83–91. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bamoharram, F.F. Vibrational Spectra Study of the Interactions Between Keggin Heteropolyanions and Amino Acids. Molecules 2009, 14, 3214-3221. https://doi.org/10.3390/molecules14093214
Bamoharram FF. Vibrational Spectra Study of the Interactions Between Keggin Heteropolyanions and Amino Acids. Molecules. 2009; 14(9):3214-3221. https://doi.org/10.3390/molecules14093214
Chicago/Turabian StyleBamoharram, Fatemeh F. 2009. "Vibrational Spectra Study of the Interactions Between Keggin Heteropolyanions and Amino Acids" Molecules 14, no. 9: 3214-3221. https://doi.org/10.3390/molecules14093214




