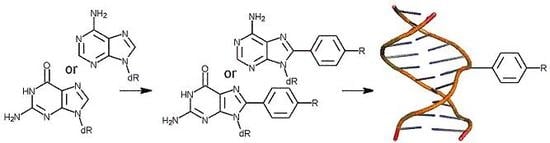
Experimental
General
2'-Deoxyguanosine was purchased from USB (Cleveland, OH, USA), tris(3-sulfonatophenyl)-phosphine hydrate sodium salt (TPPTS) was purchased from Strem Chemicals (Newburyport, MA, USA), and all other reagents were purchased from Aldrich Chemical Co (Milwaukee, WI, USA) and used as received. Solvents were purchased from Fisher Scientific (Pittsburgh, PA, USA) and were purified and dried using standard techniques. IR spectra were obtained on a Perkin-Elmer infrared spectrophotometer model 782 in potassium bromide matrix. UV spectra were obtained on a Beckman DU640 spectrophotometer. NMR spectra were obtained on a Varian Inova 600 MHz spectrometer, referenced to TMS. Proton resonances were assigned based on COSY correlations, carbons bonded to protons were assigned by Hetcor correlation, and carbons without protons were assigned on the basis of chemical shift. In the case of multiple isomers the reported chemical shifts refer to the major isomer. Mass spectra (MS) were recorded on a Finnigan LCQ Deca using electrospray ionization.
General Procedure for the Suzuki Coupling Reaction
Pd(OAc)2 (11 mg, 0.05 mmol), tris(3-sulfonatophenyl)phosphine trisodium (TPPTS; 76 mg, 0.13 mmol), Na2CO3 (400 mg, 3.77 mmol), compound 1 (0.657 g, 1.90 mmol) and aqueous acetonitrile (20 mL, 33%, pre-sparged with nitrogen) were heated at 80 °C under nitrogen. The arylboronic acid (2, 2.35 mmol) was then added and the reaction heated at 80 °C, with stirring, for 2.5 hours (ratio Pd(OAc)2:TPPTS:Na2CO3:1:2 = 1:2.6:75:38:47). The reaction mixture was cooled to room temperature, water (40 mL) added, the pH adjusted to 7 with 10% KH2PO4 or HCl, cooled in an ice bath, and filtered. The filtercake was dried in vacuo to yield the arylated product.
C8-Phenyl-2'-deoxyguanosine (3a): Yield 83%; IR (KBr) cm-1 3600-3000, 2930, 1740-1555, 1360, 1100; 1H-NMR (DMSO-d6) δ ppm 10.80 (1H, bs, NH), 7.65 (2H, dd, J = 1.8, 7.3 Hz, ArH-3,5), 7.52 (3H, m, ArH-2,4,6), 6.42 (2H, bs, NH2), 6.07 (1H, dd, J = 6.6, 7.8 Hz, H-1'), 5.14 (1H, d, J = 2.4 Hz, 3'-OH), 4.99 (1H, bs, 5'-OH), 4.33 (1H, m, H-3'), 3.79 (1H, m, H-4'), 3.65 (1H, m, H-5"), 3.54 (1H, m, H-5'), 3.17 (1H, p, J = 6.9 Hz, H-2"), 2.02 (1H, ddd, J = 2.4, 6.6, 13.2 Hz, H-2'); 13C-NMR (DMSO-d6) δ ppm 156.8, 153.1, 152.0, 147.1, 130.3, 129.4, 129.1, 128.6, 117.2, 87.9, 84.7, 71.2, 62.1, 36.6. UV (MeCN) λmax (log ε) 279 (4.4); MS m/z 344 (MH+), MS/MS 344Ψ 228 (C11H10N5O+).
C8-p-Tolyl-2’-deoxyguanosine (3b): Yield 99%; IR (KBr) cm-1 3600-3260, 3225, 2910, 1690, 1620, 1350, 1080, 1015; 1H-NMR (DMSO-d6) δ ppm 10.73 (1H, s, NH), 7.53 (2H, d, J = 8.40, ArH-3,5), 7.34 (2H, d, J = 7.80, ArH-2,6), 6.37 (2H, bs, NH2), 6.05 (1H, dd, J = 6.6, 7.8 Hz, H-1'), 5.13 (1H, d, J = 4.20, 3'-OH), 4.98 (1H, t, J = 6.00, 5'-OH), 4.33 (1H, h, J = 3.1 Hz, H-3'), 3.78 (1H, m, H-4'), 3.65 (1, p, J = 5.4 Hz, H-5"), 3.54 (1H, J = 5.4 Hz, H-5'), 3.16 (1H, p, J = 6.9 Hz, H-2"), 2.38 (3H, s, Ar-CH3), 2.00 (1H, ddd, J = 2.4, 6.6, 9 Hz, H-2'); 13C-NMR (DMSO-d6) δ ppm 156.6, 152.9, 151.9, 147.2, 139.1, 129.2, 129.0, 127.5, 117.1, 87.9, 84.6, 71.2, 62.1, 36.5, 20.9; UV (MeCN) λmax (log ε) 282 (4.5); MS: m/z 358 (MH+), MS/MS 358Ψ242 (C12H12N5O+).
C8-(4-Carboxyphenyl)-2′-deoxyguanosine (3c): Yield 83%; 1H-NMR (DMSO-d6): δ ppm 10.82 (1H, s, NH), 8.07 (2H, d, J = 7.8 Hz, phenyl), 7.80 (2H, d, J = 8.4 Hz, phenyl), 6.48 (2H, s, NH2), 6.10 (1H, t, J = 7.2 Hz, H-1′), 5.14 (1H, brs, 3′-OH), 4.96 (1H, brs, 5′-OH), 4.34 (1H, m, H-3′), 3.80 (1H, m, H-4′), 3.66 and 3.55 (2H, m, H-5′/5′′), 3.11 (1H, m, H-2′′) and 2.05 (1H, ddd, J = 3.0, 6.6, 13.2 Hz, H-2’); 13C-NMR (DMSO-d6): δ ppm 166.9, 156.6, 153.2, 152.3, 146.1, 134.3, 131.4, 129.5, 129.2, 117.4, 87.9, 84.5, 71.0, 61.9, and 36.7. UV: ε227 = 13,745 cm-1M-1 and ε285 = 18,054 cm-1M-1. MS: m/z for MW 387.35, calculated MH+ 388.35, found 388, 410 (M+Na)+, 426 (M+K)+, 775 (2M+H)+,and 797 (2M+Na)+.
C8-(4-(TBS-O-methyl)phenyl)-2′-deoxyguanosine (
3d): Yield 72%; The TBS protected boronic acid was prepared as described by Zheng,
et al. [
13];
1H-NMR (DMSO-d
6): δ ppm 10.78 (1H, s, NH), 7.62 (2H, d,
J = 8.4 Hz, phenyl), 7.46 (2H, d,
J = 8.4 Hz, phenyl), 6.39 (2H, s, NH
2), 6.06 (1H, t,
J = 7.5 Hz, H-1′), 5.12 (1H, d,
J = 3.6 Hz, 3′-OH), 4.96 (1H, d,
J=4.2 Hz, 5′-OH), 4.80 (2H, s, CH
2), 4.34 (1H, m, H-3′), 3.79 (1H, m, H-4′), 3.65 and 3.55 (2H, m, H-5′/5′′), 3.16 (1H, m, H-2′′), 2.02 (1H, m, H-2′), 0.93 (9H, s, t-butyl), and 0.11 (6H, s, dimethylsilyl);
13C-NMR (DMSO-d
6): δ ppm 156.7, 153.0, 151.9, 147.0, 142.5, 129.0, 128.9, 126.0, 117.1, 87.9, 84.7, 71.2, 63.9, 62.1, 36.6, 25.8, 18.0, and -5.3. UV: ε
281 = 15,285 cm
-1M
-1. MS: m/z for MW 487.63, calculated MH
+ 488.63, found 488, 510 (M+Na)
+, 526 (M+K)
+, 975 (2M+H)
+, and 997 (2M+Na)
+.
C8-(4-Methoxymethylphenyl)-2′-deoxyguanosine (3e): Yield 67%; 1H-NMR (DMSO-d6): δ ppm 10.76 (1H, s, NH), 7.63 (2H, d, J = 8.4 Hz, phenyl), 7.47 (2H, d, J = 7.8 Hz, phenyl), 6.39 (2H, s, NH2), 6.07 (1H, dt, J = 1.8, 7.5 Hz, H-1′), 5.12 (1H, d, J = 4.2 Hz, 3′-OH), 4.97 (1H, t, J = 5.7 Hz, 5′-OH), 4.49 (2H, s, CH2), 4.33 (1H, m, H-3′), 3.79 (1H, m, H-4′), 3.65 and 3.54 (2H, m, H-5′/5′′), 3.34 (3H, s, OCH3), 3.15 (1H, m, H-2′′) and 2.02 (1H, m, H-2′); 13C-NMR (DMSO-d6): δ ppm 156.6, 153.0, 152.0, 146.9, 139.7, 129.4, 129.1, 127.5, 117.1, 87.8, 84.6, 73.1, 71.2, 62.1, 57.7, and 36.6. UV: ε221 = 9,377 cm-1M-1 and ε282 = 15,552 cm-1M-1. MS: m/z for MW 387.40, calculated MH+ 388.40, found 411 (M+Na)+ and 798 (2M+Na)+.
C8-Phenyl-2'-deoxyadenosine (8a): Yield 85%; IR (KBr) cm-1 3500-3000, 2935, 1700-1550, 1480, 1450, 1340, 1300, 1250, 1135, 1100, 1060; 1H-NMR (DMSO-d6) δ ppm 8.15 (1H, s, H-2), 7.72 (2H, m, ArH-3,5), 7.60 (3H, m, ArH-2,4,6), 7.44 (2H, bs, NH2), 6.16 (1H, dd, J=6.6, 8.4 Hz, H-1'), 5.55 (1H, bs, 5'-OH), 5.23 (1H, bs, 3'-OH), 4.46 (1H, d, J = 4.8Hz, H-3'), 3.88 (1H, h, J =2.2 Hz, H-4'), 3.70 (1H, dd, J =4.2, 12, H-5"), 3.54 (1H, bd, J =12, H-5'), 3.31 (1H, m, H-2"), 2.15 (1H, ddd, J=1.8, 6.6, 13.2 H-2'); 13C NMR (DMSO-d6) δ ppm 156.1, 152.0, 150.4, 149.9, 130.1, 129.7, 129.5, 128.8, 119.2, 88.4, 85.7, 71.5, 62.3, 37.2; UV (MeCN) λmax (log e) 276 (4.12); MS: m/z 328 (MH+), MS/MS 328Ψ212 (C11H10N5+).
C8-p-Tolyl-2'-deoxyadenosine (8b): Yield 80%; IR (KBr) cm-1 3700-2990, 2940, 1655, 1605, 1480, 1335, 1305, 1095; 1H NMR (DMSO-d6) δ ppm 8.14 (1H, s, H-2), 7.60 (2H, d, J = 8.4 Hz, ArH-3,5), 7.40 (2H, d, J =8.4 Hz, ArH-2,6), 6.14 (1H, dd, J = 6.0, 8.4 Hz, H-1'), 5.57 (1H, bs, 5'-OH), 5.22 (1H, bs, 3'-OH), 4.46 (1H, m, H-3'), 3.87 (1H, h, J = 2.2 Hz, H-4'), 3.70 (1H, dd, J = 2.1, 12 Hz, H-5"), 3.54 (1H, bm, H-5'), 3.30 (1H, m, H-2"), 2.41 (3H, s Ar-CH3), 2.14 (1H, ddd, J = 1.8, 6, 12.6 Hz, H-2'); 13C-NMR (DMSO-d6) δ ppm 156.1, 151.8, 150.6, 149.9, 139.9, 126.8, 129.3, 129.3, 119.1, 88.4, 85.7, 71.5, 62.4, 37.2, 21.0; UV (MeCN) λmax (log ε) 279 (4.28); MS: m/z 342 (MH+), MS/MS 342Ψ226 (C12H12N5+).
General Procedure for Dimethylformamidine Protection
The arylpurine 3 (0.35 g, 1.02 mmol) was co-evaporated with dry pyridine (3 × 2 mL) dissolved in MeOH (7 mL) and N,N-dimethylformamidine dimethyl acetal (625 uL, 5.0 mmol) then added (ratio 3 (mmol):MeOH (mL):acetal (mmol) = 1:7:5). The reaction mixture was stirred at room temperature for 48 hours and then concentrated in vacuo to give 4.
N2-(N,N-Dimethylformamidine)-C8-phenyl-2'-deoxyguanosine (4a): Yield 92%; IR (KBr) cm-1 3600-3000, 2930, 1680, 1645, 1430, 1345, 1115; 1H-NMR (DMSO-d6) δ ppm 11.43 (1H, bs, NH), 8.51 (1H, s, CHN), 7.66 (2H, dd, J=1.0, 7.8 Hz, ArH-3,5), 7.55 (3H, m, ArH-2,4,6), 6.11 (1H, t, J = 6.6 Hz, H-1'), 5.21 (1H, d, J = 4.80 Hz, 3'-OH), 4.89 (1H, t, J = 4.8 Hz, 5-'OH), 4.43 (1H, q, J = 4.0 Hz, H-3'), 3.81 (1H, q, J = 3.0 Hz, H-4'), 3.67 (1H, m, H-5"), 3.57 (1H, m, H-5'), 3.22 (1H, h, J = 7.2 Hz, H-2"), 3.16 (3H, s, CH3N), 3.05 (3H, s, CH3N), 2.08 (1H, ddd, J = 2.4, 6.6, 13.2 Hz, H-2'); 13C-NMR (DMSO-d6) δ ppm 158.1, 157.5, 156.8, 152.1, 150.3, 130.2, 129.5, 129.1, 128.7, 120.2, 87.7, 84.8, 71.0, 62.0, 40.8, 37.0, 34.6; UV (MeCN) λmax (log ε) 317 (4.49) MS m/z 399 (MH+), MS/MS 399Ψ283 (C14H15N6O+).
N2-(N,N-Dimethylformamide)-C8-p-tolyl-2'-deoxyguanosine (4b): Yield 89%; IR (KBr) cm-1 3600-2700, 1675, 1630, 1570-1500, 1430, 1350, 1290; 1H-NMR (DMSO-d6) δ ppm 11.42 (1H, s, NH), 8.50 (1H, s, HCN), 7.54 (2H, d, J = 7.2 Hz, ArH-3,5), 7.36 (2H, d, J = 7.2 Hz, ArH-2,6), 6.09 (1H, m, H-1'), 5.23 (1H, m, 3'-OH), 4.91 (1H, m, 5'-OH), 4.43 (1H, m, H-3'), 3.81 (1H, m, H-4'), 3.66 (1H, m, H-5"), 3.57 (1H, m, H-5'), 3.22 (1H, m, H-2"), 3.15 (3H, s, CH3N), 3.05 (3H, s, CH3N), 2.39 (3H, s, Ar-CH3), 2.06 (1H, m, H-2'); 13C-NMR (DMSO-d6) δ ppm 158.1,157.6, 156.8, 150.6, 148.2, 139.2, 129.2, 129.0, 127.3, 120.1, 87.7, 84.9, 71.0, 62.0, 40.8, 37.0, 34.6, 20.9; UV (MeCN) λmax (log ε) 316 (4.29). MS: m/z 413 (MH+), MS/MS 413Ψ297 (C15H17N6O+).
N2-(N,N-Dimethylformamidine)-C8-(4-carboxyphenyl)-2′-deoxyguanosine (4c): Yield 96%; 1H-NMR (DMSO-d6): δ ppm 8.53 (1H, s, HC=N), 8.02 (2H, d, J = 8.4 Hz, phenyl), 7.64 (2H, d, J = 8.4 Hz, phenyl), 6.14 (1H, t, J = 7.2 Hz, H-1′), 4.45 (1H, m, H-3′), 3.82 (1H, m, H-4′), 3.68 and 3.58 (2H, m, H-5′/5′′), 3.18 (1H, m, H-2′′), 3.16 and 3.05 (3H each, s, N(CH3)2), and 2.10 (1H, ddd, J = 3.0, 6.6, 13.2 Hz, H-2′); 13C-NMR (DMSO-d6): δ ppm 169.2, 158.1, 156.8, 150.8, 148.0, 138.7, 131.2, 129.1, 128.5, 120.2, 87.7, 84.7, 71.0, 61.9, 40.8, 37.1, 34.6, and 34.1. UV: ε231 = 17,153 cm-1M-1 and ε318 = 20,164 cm-1M-1; MS: m/z for MW 442.43, calculated MH+ 443.43, found 443, 465 (M+Na)+, 488 (M+2Na)+, 885 (2M+H)+, and 907 (2M+Na)+.
N2-(N,N-Dimethylformamidine)-C8-(4-(TBS-O-methyl)phenyl)-2′-deoxyguanosine (4d): Yield 77%; 1H-NMR (DMSO-d6): δ ppm 11.42 (1H, s, NH), 8.51 (1H, s, HC=N), 7.63 (2H, d, J = 7.8 Hz, phenyl), 7.48 (2H, d, J = 7.8 Hz, phenyl), 6.11 (1H, t, J = 7.2 Hz, H-1′), 5.20 (1H, d, J = 2.4 Hz, 3′-OH), 4.88 (1H, m, 5′-OH), 4.81 (2H, s, CH2), 4.44 (1H, m, H-3′), 3.82 (1H, m, H-4′), 3.67 and 3.57 (2H, m, H-5′/5′′), 3.22 (1H, m, H-2′′), 3.16 and 3.05 (3H each, s, N(CH3)2), 2.08 (1H, m, H-2′), 0.93 (9H, s, t-butyl), and 0.12 (6H, s, dimethylsilyl); 13C-NMR (DMSO-d6): δ ppm 158.1, 157.5, 156.8, 150.6, 148.0, 142.7, 129.0, 128.7, 126.0, 120.2, 87.7, 84.8, 71.0, 63.9, 62.0, 40.8, 37.0, 34.6, 25.8, 18.0, and -5.4. UV: ε229 = 21,603 cm-1M-1 and ε313 = 28,129 cm-1M-1; MS: m/z for MW 542.71, calculated MH+ 543.71, found 543, 565 (M+Na)+, 1085 (2M)+, and 1107 (2M+Na)+.
N2-(N,N-Dimethylformamidine)-C8-(4-methoxymethylphenyl)-2′-deoxyguanosine (4e): Yield 90%; 1H-NMR (DMSO-d6): δ ppm 11.45 (1H, s, NH), 8.51 (1H, s, HC=N), 7.64 (2H, d, J = 8.4 Hz, phenyl), 7.49 (2H, d, J = 7.8 Hz, phenyl), 6.12 (1H, t, J = 7.2 Hz, H-1′), 5.21 (1H, d, J = 4.2 Hz, 3′-OH), 4.88 (1H, dt, J = 1.8, 6.0 Hz, 5′-OH), 4.50 (2H, s, CH2), 4.44 (1H, m, H-3′), 3.82 (1H, m, H-4′), 3.68 and 3.57 (2H, m, H-5′/5′′), 3.34 (3H, s, OCH3 ), 3.22 (1H, m, H-2′′), 3.16 and 3.05 (3H each, s, N(CH3)2), and 2.08 (1H, m, H-2′); 13C-NMR (DMSO-d6): δ ppm 158.1, 157.5, 156.8, 150.7, 147.9, 139.9, 129.2, 129.1, 127.6, 120.2, 87.7, 84.8, 73.1, 71.0, 62.0, 57.7, 40.8, 37.0, and 34.6. UV: ε229 = 14,602 cm-1M-1 and ε314 = 20,834 cm-1M-1; MS: m/z for MW 442.47, calculated MH+ 443.47, found 443, 466 (M+Na)+, and 908 (2M+Na)+.
N6-(N,N-Dimethylformamide)-C8-phenyl-2'-deoxyadenosine (9a): Yield 64%; IR (KBr) cm-1 3650-3000, 2935, 1640, 1570, 1450, 1420, 1340, 1100; 1H-NMR (DMSO-d6) δ ppm 8.93 (1H, s, HCN), 8.43 (1H, s, H-2), 7.76 (2H, m, ArH-3,5), 7.62 (3H, m, ArH-2,4,6), 6.19 (1H, dd, J = 6.6, 8.4, H-1'), 5.34 (1H, bs, 5'-OH), 5.27 (1H, bs, 3'-OH), 4.48 (1H, m, H-3'), 3.87 (1H, h, J = 2.3 Hz, H-4'), 3.71 (1H, bd, J = 11.4 Hz, H-5"), 3.54 (1H, m, H-5'), 3.36 (1H, m, H-2"), 3.20 (3H, s, CH3N), 3.14 (3H, s, CH3N), 2.17 (1H, ddd, J = 2.4, 6.6, 13.2 Hz, H-2'); 13C-NMR (DMSO-d6) δ ppm 159.2, 157.9, 152.2, 152.1, 151.2, 130.2, 129.7, 129.5, 128.8, 125.7, 88.2, 85.5, 71.3, 62.2, 40.7, 36.9, 34.6; UV (MeCN) λmax (log ε) 322 (4.38), 256 (4.12); MS: m/z 383 (MH+), MS/MS 383Ψ267 (C14H15N6+).
N6-(N,N-Dimethylformamide)-C8-p-tolyl-2'-deoxyadenosine (9b): Yield 97%; IR (KBr) cm-1 3500-3100, 2920, 1630, 1570, 1460, 1420, 1330, 1110, 1090, 1050; 1H-NMR (DMSO-d6) δ ppm 8.92 (1H, s, HCN), 8.42 (1H, s, H-2), 7.64 (2H, d, J = 8.40, ArH-3,5), 7.42 (2H, d, J = 8.4, ArH-2,6), 6.18 (1H, t, J =7.2 Hz, H-1'), 5.37 (1H, bs, 5'-OH), 5.24 (1H, bs, 3'-OH), 4.48 (1H, m, H-3'), 3.87 (1H, m, H-4'), 3.71 (1H, bd, J = 12 Hz, H-5"), 3.54 (1H, bd, J = 12 Hz, H-5'), 3.35 (1H, m, H-2"), 3.20 (3H, s, CH3N), 3.13 (3H, s, CH3N), 2.42 (3H, s, Ar-CH3), 2.15 (1H, bdd, J = 6, 13.8 Hz, H-2'); 13C-NMR (DMSO-d6) δ ppm 159.1, 157.8, 152.3, 152.0, 151.1, 126.8, 140.1, 129.3, 129.4, 125.7, 88.2, 85.5, 71.4, 62.2, 40.7, 36.9, 34.6, 21.0; UV (MeCN) λmax (log ε) 258 (4.31), 323 (4.56); MS: m/z 397 (MH+), MS/MS 397Ψ281 (C15H17N6+).
General Procedure for Dimethoxytrityl Protection
Compound 4 (0.63 mmol) was dried in vacuo (P2O5), dissolved in pyridine (PYR) (5 mL), TEA (0.11 mL) added and then 4,4'-dimethoxytritylchloride (DMTrCl) (0.314 mg, 0.93 mmol) (ratio 4 (mmol):PYR (mL):TEA (mL):DMTrCl (mmol) = 47:1:6:8.9). The reaction mixture stirred for 2.5 hours at room temperature, MeOH (3 mL) added to quench the reaction, the solvent was removed in vacuo and residual purified (alumina, MeOH:CH2Cl2, 0-10%).
5'-O-(4,4'-Dimethoxytrityl)-N2-(N,N-dimethylformamidine)-C8-phenyl-2'-deoxyguanosine (5a): Yield 75%; IR (KBr) cm-1 3600-3150, 3065, 2940, 1685, 1630, 1570-1490, 1440, 1340, 1250, 1175, 1110, 1030. 1H-NMR (acetone-d6) δ ppm 8.32 (1H, s, HCN), 7.79 (2H, m, ArH-3,5), 7.51 (3H, m, 3H, ArH-2,4,6), 7.37 (2H, d, J = 7.8 Hz, ArH-3',5'), 7.249 and 7.238 (4H, d, J = 9.0 Hz, ArH-3",5"), 7.19 (3H, m, ArH-2',4',6'), 6.752 and 6.718 (4H, d, J = 9.0 Hz, ArH-2",6"), 6.18 (1H, dd, J = 5.4, 7.8 Hz, H-1'), 4.68 (1H, dt, J = 5.4, 7.2 Hz, H-3'), 3.98 (1H, m, H-4'), 3.73 (3H, s, CH3O), 3.72 (3H, s, CH3O), 3.40 (1H, dd, J = 7.2, 9.9 Hz, H-5"), 3.34 (1H, m, H-2"), 3.20 (1H, dd, J = 3.6, 9.9 Hz, H-5'), 3.03 (3H, s, CH3N), 2.95 (3H, s, CH3N), 2.17 (1H, m, H-2'); 13C-NMR (acetone-d6) δ ppm 159.6, 159.0, 158.7, 157.4, 152.1, 150.3, 146.4, 137.3, 137.0, 131.7, 131.1, 130.6, 130.5, 129.7, 129.1, 128.8, 127.8, 121.6, 114.0, 87.3, 85.8, 72.8, 65.5, 56.0, 55.9, 41.8, 38.4, 35.4; UV (MeCN) λmax (log ε) 320 (4.44); MS: m/z 701 (MH+), MS/MS 701Ψ303 (C21H19O2+), MS/MS 701Ψ283 (C14H15N6O+).
5'-O-(4,4'-Dimethoxytrityl)-N2-(N,N-dimethylformamidine)-C8-p-tolyl-2'-deoxyguanosine (5b): Yield: 27%; IR (KBr) cm-1 2940, 1690, 1635, 1510, 1250, 1175, 1030; 1H-NMR (MeCN-d3) δ ppm 8.31 (1H, s, HCN), 7.63 (2H, d, J = 8.0 Hz, ArH-3,5), 7.36 (2H, d, J = 7.9 Hz, DMTrH-3',5'), 7.29 (2H, d, J = 8.0 Hz, ArH-2,6), 7.24 and 7.23 (4H, m, DMTrH-3’,3",5’,5"), 7.18 (2H, m, DMTrH-2,6), 7.16 (1H, m, DMTrH-4'), 6.74 and 6.71 (4H, d, J = 6.72 Hz, DMTrH-2’,2",6’,6"), 6.17 (1H, dd, J = 5.4, 8.0 Hz, H-1'), 4.68 (1H, q, J = 5.7 Hz, H-3'), 3.98 (1H, ddd, J = 3, 5, 7.5 Hz, H-4'), 3.71 (3H, s, CH3O), 3.70 (3H, s, CH3O), 3.39 (1H, dd, J = 7.5, 9.9 Hz, H-5"), 3.33 (1H, ddd, J = 5.0, 7.1, 13.3 Hz, H-2''), 3.20 (1H, dd, J = 3.0, 9.9 Hz, H-5'), 3.00 (3H, s, CH3N), 2.93 (3H, s, CH3N), 2.40 (3H, s, Ar-CH3), 2.17 (1H, ddd, J =5.7, 8.2, 13.3 Hz, H-2'); 13C-NMR (MeCN-d3) δ ppm 159.5, 158.9,158.8, 157.2, 152.0, 150.4, 146.5, 140.9, 137.2, 137.0, 130.4, 131.0, 130.0, 129.0, 128.7, 128.0, 127.8, 121.2, 114.0, 87.2, 85.7, 72.8, 65.5, 56.0, 55.9, 41.8, 38.3, 35.3, 21.5; UV (MeCN) λmax (log ε) 322 (4.41), 231 (4.62); MS: m/z 715 (MH+), MS/MS 715Ψ303 (C21H19O2+), MS/MS 715Ψ297 (C15H17N6O+).
5′-O-(DMTr)-N2-(N,N-dimethylformamidine)-C8-(4-carboxyphenyl)-2′-deoxyguanosine (5c): Yield 31%; 1H-NMR (DMSO-d6): δ ppm 8.32 (1H, s, HC=N), 8.05 (2H, d, J = 7.8, phenyl), 7.83 (2H, d, J = 8.4, phenyl), 7.32 (2H, d, J = 8.2 Hz, DMTr-H), 7.17-7.2 (3H, m, DMTrH-3,4,5), 7.19 (2H, d, J = 7.8 Hz, DMTrH-3’,5’), 7.18 (2H, d, J = 7.8 Hz, DMTrH-3”,5”), 6.77 (2H, d, J = 7.8 Hz, DMTrH-2”,6”), 6.73 (2H, d, J = 7.8 Hz, DMTrH-2’,6’), 6.20 (1H, dd, J = 4.8, 7.8, H-1′), 4.57 (1H, q, J = 5.9 Hz, H-3′), 3.93 (1H, m, H-4′), 3.71 and 3.70 (3H each, s, OCH3), 3.30 (1H, dd, J = 7.2,10 Hz, H-5”), 3.21 (1H, ddd, J = 5.4, 7.2, 13.4 Hz, H-2′′), 3.15 (1H, dd, J = 2.8,10 Hz, H-5′), 3.03 and 2.98 (3H each, s, N(CH3)2), and 2.20 (1H, ddd, J = 6.0,8.0,13.4 Hz, H-2′); 13C-NMR (DMSO-d6): δ ppm 157.9, 157.9, 157.6, 157.5, 156.4, 150.5, 147.5, 144.9, 135.7, 135.6, 129.6, 129.4, 129.3, 129.0, 127.6, 127.6, 126.5, 120.3, 112.9, 112.9, 85.7, 85.2, 84.1, 70.8, 64.0, 54.9, 54.9, 40.7, 37.4, 34.6, and 34.2; UV: (MeCN) λmax (log ε) 233 (4.63) and 322 (4.44); MS: m/z for MW 744.80, calculated MH+ 745.80, found 745.767 (M+Na)+, and 1511.0 (2M+Na)+.
5′-O-(DMTr)-N2-(N,N-dimethylformamidine)-C8-(4-(TBS-O-methyl)phenyl)-2′-deoxyguanosine (5d): Yield 41%; 1H-NMR (DMSO-d6): δ ppm 11.41 (1H, s, NH), 8.31 (1H, s, HC=N), 7.74 and 7.46 (4H, d, J = 8.4, ArH-2,6 and ArH-3,5), 7.33 (2H, d, J = 8.4 Hz, DMTrH-2,6), 7.24 (3H, m, DMTrH-3,4,5), 7.21 (2H, d, J = 8.5 Hz, DMTrH-3’,5’), 7.18 (2H, d, J = 8.5 Hz, DMTrH-3”,5”), 6.77 (2H, d, J = 8.5 Hz, DMTrH-2’,6’), 6.73 (2H, d, J = 8.5 Hz, DMTrH-2”,6”), 6.17 (1H, dd, J = 5.4,7.8 Hz, H-1′), 5.28 (1H, d, J = 5.6 Hz, 3′-OH), 4.81 (2H, s, CH2), 4.57 (1H, p, J = 5.1 Hz, H-3′), 3.93 (1H, m, H-4′), 3.71 and 3.70 (3H each, s, OCH3), 3.32 (1H, dd, J = 6.3, 10.2 Hz, H-5’or 5′′), 3.21 (1H, p, J = 6.3 Hz, H-2′′), 3.15 (2H, dd, J = 2.8, 10.2 Hz, H-5′ or 5′′), 3.03 and 2.98 (3H each, s, N(CH3)2), 2.18 (1H, ddd, J = 5.8,8,13.7, H-2′), 0.94 (9H, s, t-butyl), and 0.12 (6H, s, dimethylsilyl); 13C-NMR (DMSO-d6): δ ppm 157.9, 157.9, 157.5, 156.3, 150.4, 148.2, 144.9, 142.6, 135.7, 135.6, 129.6, 129.4, 129.1, 128.9, 127.6, 127.6, 126.5, 126.0, 120.0, 113.1, 112.9, 112.9, 85.7, 85.2, 84.0, 70.9, 64.0, 63.9, 54.9, 54.9, 40.7, 37.4, 34.6, 25.8, 18.0, and -5.4; UV: (MeCN) λmax (log ε) 231 (4.60) 316 (4.36); MS: m/z for MW 845.08, calculated MH+ 846.08, found 845, 867 (M+Na)+, and 1712 (2M+Na)+.
5′-O-(DMTr)-N2-(N,N-dimethylformamidine)-C8-(4-methoxymethylphenyl)-2′-deoxyguanosine (5e): Yield 55%; 1H-NMR (DMSO-d6): δ ppm 11.42 (1H, s, NH), 8.31 (1H, s, HC=N), 7.74 (2H, d, J = 8.4 Hz, ArH-2,6 or 3,5), 7.46 (2H, d, J = 8.4, ArH-2,6 or 3,5), 7.32 (2H, d, J =8.9 Hz, DMTrH-2,6), 7.19 (2H, d, J = 8.7 Hz, DMTrH-3’,5’), 7.18 (2H, d, J = 8.7 Hz, DMTrH-3”,5”), 7.14-7.18 (3H, m, ArH-3,4 5), 6.77 (2H, d, J = 8.7 Hz, DMTrH-2”,6”), 6.73 (2H, d, J = 8.7 Hz, DMTrH-2’,6’), 6.17 (1H, dd, J = 3.0, 6.6 Hz, H-1′), 5.28 (1H, d, J = 4.9 Hz, 3′-OH), 4.57 (1H, p, 5.5 Hz, H-3′), 4.50 (2H, s,CH2), 3.92 (1H, m, H-4′), 3.71 and 3.70 (3H each, s, OCH3), 3.35 (3H, s, CH3), 3.30 (1H, dd, J = 7,9.9 Hz, H-5′ or 5′′), 3.20 (1H, ddd, J = 5,6.8,13 Hz, H-2′′), 3.15 (1H, dd, J = 3, 9.9 Hz, H-5′ or 5′′), 3.03 and 2.98 (3H each, s, N(CH3)2), and 2.18 (1H, ddd, 5.7, 8, 13.5 Hz, H-2′); 13C-NMR (DMSO-d6): δ ppm 157.9, 157.9, 157.5, 156.3, 150.4, 148.1, 144.9, 139.8, 135.7, 135.6, 129.6, 129.4, 129.1, 127.6, 127.6, 127.5, 126.5, 120.1, 112.9, 112.9, 85.7, 85.2, 84.0, 73.1, 70.9, 64.0, 57.7, 54.9, 54.9, 40.7, 37.4, and 34.6; UV: (MeCN) λmax (log ε) 231 (4.54) and 319 (4.37); MS: m/z for MW 744.85, calculated MH+ 745.85, found 746, 768 (M+Na)+, 784 (M+K)+, and 1512 (2M+Na)+.
5'-O-(4,4'-Dimethoxytrityl)-N2-(N,N-dimethylformamidine)-C8-phenyl-2'-deoxyadenosine (10a): Yield 58%; IR (KBr) cm-1 3500-3130, 2940, 2600, 2500, 1670-1530, 1505, 1440, 1420, 1330, 1250, 1170, 1110, 1040; 1H-NMR (MeCN-d3) δ ppm 8.87 (1H, s, HCN), 8.20 (1H, s, H-2), 7.83 (2H, d, J = 7.3 Hz, ArH-3,5), 7.55 (3H, m, ArH-2,6), 7.38 (2H, d, J = 7Hz DMTrH-2”,6”), 7.25 and 7.24 (4H, d, J = 8.8 Hz, DMTrH-3’,3",5’,5"), 7.19 (3H, m, DMTrH-3”,4”,5”), 6.85 and 6.75 (4H, d, J = 8.8 Hz, DMTrH-2’,2",6’,6"), 6.21 (1H, t, J = 7.0 Hz, H-1'), 4.74 (1H, m, H- 3'), 4.07 (1H, m, H-4'), 3.73 (3H, s, CH3O), 3.70 (3H, s, CH3O), 3.53 (1H, dd, J = 6.9, 10.3 Hz, H-2"), 3.37 (1H, dd, J = 4.4, 10.3 Hz, H-5"), 3.28 (1H, dt, J = 7, 13.5 Hz, H-5'), 3.16 (3H, s, CH3N), 3.13 (3H, s, CH3N), 2.17 (1H, ddd, J = 4.2, 7.9, 13.5 Hz, H-2'). 13C-NMR (MeCN-d3) δ ppm 159.6, 159.5, 158.9, 154.2, 153.8, 152.5, 146.4, 137.3, 137.1, 137.0, 131.1, 130.8, 130.1, 129.7, 129.1, 128.7, 127.7, 124.8, 114.0, 87.4, 86.2, 73.1, 65.4, 56.0, 55.9, 41.6, 37.4, 35.3; UV(MeCN) λmax (log ε) 320 (4.25); MS: m/z 685 (MH+), MS/MS 685Ψ303 (C21H19O2+), MS/MS 685Ψ267 (C14H15N6+).
5'-O-(4,4'-Dimethoxytrityl)-N2-(N,N-dimethylformamidine)-C8-p-tolyl-2'-deoxyadenosine (10b): Yield 86%; IR (KBr) cm-1 2940, 1610, 1560, 1250, 1180, 1035; 1H-NMR (MeCN-d3) δ ppm 8.86 (1H, s, HCN), 8.21 (1H, s, H-2), 7.73 (2H, m, ArH-3,5), 7.38 (2H, m, ArH-3',5'), 7.35 (2H, m, ArH-2,6), 7.25 (4H, m, ArH-3",5"), 7.22 (2H, m, ArH-2',6'), 7.19 (1H, m, ArH-4'), 6.74 (4H, m, ArH-2",6"), 6.23 (1H, m, H-1'), 4.74 (1H, m, H- 3'), 4.05 (1H, m, H-4'), 3.71 (3H, s, CH3O), 3.70 (3H, s, CH3O), 3.52 (1H, m, H-5"), 3.34 (1H, m, H-2"), 3.29 (1H, m, H-5'), 3.14 (3H, s, CH3N), 3.13 (3H, s, CH3N), 2.41 (3H, s, ArCH3), 2.13 (1H, m, H-2'); 13C-NMR (MeCN-d3) δ ppm 159.7, 158.9, 156.7, 153.0, 152.4, 151.8, 146.4, 140.6, 137.3, 137.1, 131.1, 130.9, 130.4, 129.1, 128.7, 127.7, 127.2, 124.8, 114.0, 87.4, 86.4, 73.1, 65.3, 56.0, 55.9, 41.5, 37.4, 35.3, 21.6; UV (MeCN) λmax (log ε) 234 (4.63); MS: m/z 699 (MH+), MS/MS 699Ψ303 (C21H19O2+), MS/MS 699Ψ281 (C15H17N6+).
General Procedure for Phosphoramidite Synthesis
Compound 5 (0.29 mmol) was dried in vacuo (P2O5). CH2Cl2 (3 mL), TEA (80 μL), and 2-cyanoethyl diisopropylchlorophosphoramidite (CEDClP) (65 μL, 0.29 mmol) were added, the reaction at room temperature for 30 min, a second addition of 2-cyanoethyl diisopropylchlorophosphoramidite (32 μL, 0.14 mmol) made, and stirring at room temperature continued for 30 min (Ratio 5 (mmol):TEA (mmol):CEDClP (mmol):2nd CEDClP (mmol) for all reactions = 2:4:2:1). The reaction solvent was removed in vacuo, THF:benzene (1:4, 4 mL) added, the mixture stirred for 10 minutes, and then filtered and the filtrate concentrated in vacuo. The residual solid was purified by low pressure alumina chromatography (100% EtOAc).
3′-O-[(2-Cyanoethoxy)(diisopropylamino)phosphino]-5′-O-(4,4′-dimethoxytrityl)-N2-(N,N-dimethyl-formamidine)-C8-phenyl-2′-deoxyguanosine (6a): Yield 44%; IR (KBr) cm-1 2940, 1675, 1630, 1550-1500, 1430, 1350, 1250, 1180, 1120; 1H-NMR (acetone-d6) δ ppm 8.39 (1H, s, HCN), 7.80 (2H, m, ArH-3,5), 7.54 (3H, m, ArH-2,4,6), 7.35 (2H, m, ArH-3',5'), 7.23 (4H, m, ArH-3",5"), 7.19 (2H, m, ArH-2',6'), 7.17 (1H, m, ArH-4'), 6.74 (4H, m, ArH-2",6"), 6.21 (1H, m, H-1'), 5.04 (1H, m, H- 3'), 4.10 (1H, m, H-4'), 4.04 (2H, m, CH2O), 3.75 (3H, s, CH3O), 3.73 (3H, s, CH3O), 3.68 (1H, m, H-2"), 3.53 (2H, m, CH(CH3)2). 3.36 (1H, m, H-5"), 3.29 (1H, m, H-5'), 3.05 (3H, s, CH3N), 3.01 (3H, s, CH3N), 2.72 (2H, m, CH2CN), 2.35 (1H, m, H-2'), 1.13 (12H, m, CH(CH3)2); 13C-NMR (acetone-d6) δ ppm 159.7, 158.9, 158.6, 157.6, 151.8, 150.2, 146.1, 137.1, 137.0, 136.9, 130.8, 130.7, 130.5, 129.7), 129.0, 128.9, 127.8, 121.6, 118.4, 114.0, 87.0, 85.0, 76.0, 63.7, 61.0, 56.0, 55.9, 47.1, 41.5, 36.9, 35.5, 24.9, 21.0; UV (MeCN) λmax (log ε) 323 (4.26), 285 (4.07); MS: m/z 901 (MH+), MS/MS 901Ψ683 (C40H39N6O5+), MS/MS 901Ψ 619 (C36H45NO6P+), MS/MS 901Ψ 303 (C21H19O2+), MS/MS 901Ψ283 (C14H15N6O+).
3′-O-[(2-Cyanoethoxy)(diisopropylamino)phosphino]-5′-O-(4,4′-dimethoxytrityl)-N6-(N,N-dimethyl-formamidine)-C8-p-tolyl-2′-deoxyguanosine (6b): Yield 44%; IR (KBr) cm-1 2970, 1700, 1630, 1540, 1510, 1345, 1250, 1170, 1115, 1030; 1H-NMR (MeCN-d3) δ ppm 8.49 (1H, s, HCN), 7.79 (2H, m, ArH-3,5), 7.46 (2H, m, ArH-2,6), 7.50 (2H, m, ArH-3',5'), 7.32 (4H, m, ArH-3",5"), 7.28 (2H, m, ArH-2',6'), 7.27 (1H, m, ArH-4'), 6.85 (4H, m, ArH-2",6"), 6.31 (1H, m, H-1'), 5.16 (1H, m, H- 3'), 4.21 (1H, m, H-4'), 3.85 (3H, s, CH3O), 3.84 (3H, s, CH3O), 3.78 (2H, m, CH2O), 3.64 (2H, m, CH(CH3)2). 3.46 (1H, m, H-5"), 3.44 (1H, m, H-5'), 3.35 (1H, m, H-2"), 3.15 (3H, s, CH3N), 3.11 (3H, s, CH3N), 2.66 (2H, m, CH2CN), 2.53 (3H, s, ArCH3), 2.44 (1H, m, H-2'), 1.23 (12H, m, CH(CH3)2); 13C-NMR (MeCN-d3) δ ppm 159.6, 158.8, 158.1, 157.4, 151.9, 150.9, 146.3, 141.0, 137.2, 137.0, 131.1, 130.4, 130.3, 129.1, 128.8, 128.0, 127.8, 121.6, 119.4, 114.0, 86.9, 85.4, 77.4, 64.7, 59.5, 56.0, 55.9, 44.2, 41.9, 38.1, 35.4, 24.9, 21.1; UV (MeCN) λmax (log ε) 322 (4.44), 285 (4.27); MS: m/z 915 (MH+), MS/MS 915Ψ697 (C41H41N6O5+), MS/MS 915Ψ619 (C36H45NO6P+), MS/MS 915Ψ303 (C21H19O2+), MS/MS 915Ψ297 (C15H17N6O+).
3′-O-[(2-Cyanoethoxy)(diisopropylamino)phosphino]-5′-O-(4,4′-dimethoxytrityl)-N6-(N,N-dimethyl-formamidine)-C8-phenyl-2′-deoxyadenosine (11a): Yield 90%; IR (KBr) cm-1 2970, 1610, 1560, 1510, 1440, 1415, 1340, 1245, 1175, 1030; 1H-NMR (CDCl3) δ ppm 8.94 (1H, s, HCN), 8.31 (1H, s, H-2), 7.89 (2H, m, ArH-3,5), 7.51 (3H, m, ArH-2,4,6), 7.43 (2H, m, ArH-3',5'), 7.31 (4H, m, ArH-3",5"), 7.20 (2H, m, ArH-2',6'), 7.17 (1H, m, ArH-4'), 6.75 (4H, m, ArH-2",6"), 6.23 (1H, m, H-1'), 5.06 (1H, m, H- 3'), 4.30 (1H, m, H-4'), 3.78 (3H, s, CH3O), 3.76 (3H, s, CH3O), 3.74 (2H, m, CH2O), 3.68 (1H, m, H-2"), 3.56 (2H, m, CH(CH3)2), 3.51 (1H, m, H-5"), 3.44 (1H, m, H-5'), 3.26 (3H, s, CH3N), 3.20 (3H, s, CH3N), 2.54 (2H, m, CH2CN), 2.32 (1H, m, H-2'), 1.18 (12H, m, CH(CH3)2); 13CNMR (CDCl3) δ ppm 159.3, 158.4, 158.1, 153.5, 152.8, 151.9, 145.0, 136.4, 136.3, 136.2, 130.3, 130.2, 130.1, 128.7, 128.4, 127.8, 126.8, 117.5, 117.0, 113.0, 85.6, 85.3, 74.8, 58.5, 55.5, 55.4, 45.6, 43.5, 41.5, 36.2, 35.5, 24.8, 20.5; UV(MeCN) λmax (log ε) 321 (4.33); MS: m/z 885 (MH+), MS/MS 885Ψ667 (C40H39N6O4+), MS/MS 885Ψ619 (C36H45NO6P+), MS/MS 885Ψ303 (C21H19O2+), MS/MS 885Ψ267 (C14H15N6+).
3′-O-[(2-Cyanoethoxy)(diisopropylamino)phosphino]-5′-O-(4,4′-dimethoxytrityl)-N6-(N,N-dimethyl-formamidine)-C8-p-tolyl-2′-deoxyadenosine (11b): Yield 17%; IR (KBr) cm-1 2975, 1570, 1510, 1420, 1340, 1250, 1175, 1030; 1H-NMR (MeCN-d3) δ ppm 8.87 (1H, s, HCN), 8.25 (1H, s, H-2), 7.74 (2H, m, ArH-3,5), 7.38 (2H, m, ArH-3',5'), 7.36 (2H, m, ArH-2,6), 7.24 (4H, m, ArH-3",5"), 7.20 (2H, m, ArH-2',6'), 7.18 (1H, m, ArH-4'), 6.75 (4H, m, ArH-2",6"), 6.25 (1H, m, H-1'), 5.12 (1H, m, H- 3'), 4.17 (1H, m, H-4'), 4.07 (2H, m, CH2O), 3.73 (3H, s, CH3O), 3.71 (3H, s, CH3O), 3.68 (1H, m, H-2"), 3.55 (2H, m, CH(CH3)2), 3.38 (1H, m, H-5"), 3.31 (1H, m, H-5'), 3.18 (3H, s, CH3N), 3.15 (3H, s, CH3N), 2.53 (2H, m, CH2CN, 2.48 (1H, m, H-2'), 2.43 (3H, s, ArCH3), 1.16 (12H, m, CH(CH3)2); 13C- NMR (MeCN-d3) δ ppm 159.6, 158.9, 154.2, 153.6, 152.5, 152.2, 146.3, 141.6, 137.4, 137.1, 131.0, 130.7, 130.4, 129.0, 128.7, 128.5, 127.7, 127.0, 119.5, 113.9, 86.4, 85.9, 74.5, 64.9, 59.7, 56.0, 55.9, 44.0, 41.5, 37.0, 35.2, 25.0, 21.6, 21.1; UV(MeCN) λmax (log ε) 321 (4.47), 235 (4.53); MS: m/z 889 (MH+), MS/MS 899Ψ681 (C41H41N6O4+), MS/MS 899Ψ619 (C36H45NO6P+), MS/MS 899Ψ303 (C21H19O2+), MS/MS 899Ψ281 (C15H17N6+).