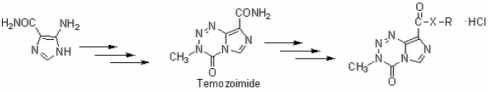
Synthesis and Antitumor Activity of 3-Methyl-4-oxo-3,4-dihydroimidazo [5,1-d][1,2,3,5]tetrazine-8-carboxylates and -carboxamides
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry

2.2. Antiproliferative activities
| Compd. | X | R | Survival percent / %a) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC-3 | LNCaP | T47D | MDA-MB-231 | DU145 | HT29 | HCT-15 | |||
| IIIa | O | -CH2CH2N(CH3)2. HCl | 87.98±0.40 | 89.37±1.75 | 66.40±1.07 | 74.61±1.61 | 92.60±1.37 | 93.89±1.28 | 92.30±0.69 |
| IIIb | O | -CH2CH2N(C2H5)2. HCl | 92.10±1.42 | 81.89±1.45 | 63.65±1.41 | 70.36±1.50 | 92.70±2.05 | 86.22±1.66 | 82.00±1.93 |
| IIIc | O | -(CH2)3N(CH3)2. HCl | 114.86±0.52 | 91.33±0.94 | 81.13±0.35 | 76.51±1.03 | 90.15±1.70 | 79.92±1.59 | 95.59±0.49 |
| IIId | O | -(CH2)3N(C2H5)2 . HCl | 88.46±0.96 | 75.95±0.41 | 62.93±1.40 | 65.70±0.59 | 74.97±1.24 | 70.96±0.92 | 91.95±1.58 |
| IIIe | O | -CH(CH3)CH2N(CH3)2 . HCl | 86.92±1.41 | 85.28±0.67 | 73.06±2.04 | 73.97±1.31 | 129.12±1.72 | 92.62±0.97 | 88.13±0.77 |
| IIIf | O | -CH(CH3)CH2N(C2H5)2. HCl | 94.43±0.75 | 76.83±1.19 | 78.84±0.35 | 80.64±0.94 | 87.47±1.90 | 84.55±1.68 | 93.70±1.54 |
| IIIg | O |  | 76.95±0.67 | 68.62±1.20 | 53.67±1.69 | 58.17±0.98 | 73.98±0.54 | 75.19±0.74 | 81.96±1.10 |
| IIIh | O | -CH(CH3)CH2N(CH3)2 . HCl | 78.07±1.71 | 75.03±1.28 | 53.50±1.22 | 68.57±0.84 | 54.61±1.28 | 66.48±0.91 | 81.99±0.94 |
| IVa | NH | -CH2CH2N(CH3)2. HCl | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| IVb | NH | -CH2CH2N(C2H5)2. HCl | 63.80±1.87 | 91.73±1.01 | 71.70±1.35 | 58.78±0.86 | 87.11±0.87 | 82.59±1.23 | 126.86±2.90 |
| IVc | NH | -(CH2)3N(CH3)2. HCl | 74.82±1.79 | 86.17±0.86 | 78.90±1.05 | 66.13±1.39 | 181.76±1.60 | 84.26±1.20 | 91.70±1.10 |
| IVd | NH | -(CH2)3N(C2H5)2. HCl | 80.20±1.71 | 78.66±0.60 | 50.94±0.46 | 53.14±2.42 | 175.59±1.54 | 61.00±3.91 | 101.83±1.20 |
| IVe |  | 88.19±1.21 | 96.05±0.70 | 96.01±0.76 | 71.27±1.18 | 162.14±1.48 | 81.99±1.34 | 94.81±0.78 | |
| IVf |  | 79.63±0.42 | 115.79±4.25 | 67.08±0.75 | 67.09±1.30 | 99.92±1.37 | 76.28±1.15 | 90.64±0.83 | |
| IVg | NH |  | 64.24±1.22 | 73.56±0.87 | 48.29±1.27 | 62.58±1.43 | 54.12±1.70 | 87.42±0.54 | 91.90±3.14 |
| IVh | NH |  | 64.16±0.76 | 55.78±0.60 | 32.65±0.63 | 54.94±1.12 | 32.77±2.35 | 83.06±1.76 | 88.47±1.40 |
| IVi | NH |  | 62.89±2.46 | 59.61±0.87 | 34.97±0.68 | 59.39±1.14 | 52.12±0.68 | 93.25±0.82 | 73.44±0.99 |
| I | -OH | 70.94±2.75 | 62.84±5.80 | 59.22±1.71 | 70.53±0.45 | 71.94±1.44 | 77.08±1.90 | 97.57±1.04 | |
| Tem | -NH2 | 85.13±2.97 | 68.51±1.25 | 62.35±0.54 | 86.85±1.67 | 70.67±1.02 | 85.20±0.93 | 76.03±1.38 | |
| Compd. | IC50(μmol/mL)a) | Compd. | IC50(μmol/mL)a) | Compd. | IC50(μmol/mL)a) |
|---|---|---|---|---|---|
| IIIa | 12.11±1.21 | IVa | >80 | IVg | 24.95±0.17 |
| IIIb | 3.24±0.09 | IVb | >80 | IVh | 70.74±2.57 |
| IIIc | 3.32±0.16 | IVc | >80 | IVi | 79.50±1.22 |
| IIId | 2.80±0.26 | IVd | >80 | I | 45.47±0.35 |
| IIIe | 2.63±0.24 | IVe | >80 | Tem | >80 |
| IIIf | 3.18±0.07 | IVf | 44.84±0.30 |
| Compd. | A1/A0 a) | Compd. | A1/A0 a) | Compd. | A1/A0 a) |
|---|---|---|---|---|---|
| IIIa | 88±2 | IIIg | 107±3 | IVe | 85±3 |
| IIIb | 97±4 | IIIh | 98±2 | IVf | 83±2 |
| IIIc | 78±2 | IVa | 76±2 | IVg | 35±3 |
| IIId | 93±2 | IVb | 64±4 | IVh | 36±4 |
| IIIe | 103±3 | IVc | 77±3 | IVi | 13±3 |
| IIIf | 91±3 | IVd | 62±3 |
3. Experimental
3.1. General
3.2. Biological activity assays
3.3. Solubility detection
4. Conclusions
Acknowledgements
- Sample Availability: Contact the authors.
References and Notes
- Stevens, M.F.G.; Hickman, J.A.; Langdon, S.P.; Chubb, D.; Vickers, L.; Stone, R.; Baig, G.; Goddard, C.; Gibson, N.W.; Slack, J.A.; Newton, C.; Lunt, E.; Fizames, C.; Lavelle, F. Antitumor Activity and Pharmacokinetics in Mice of 8-Carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a Novel Drug with Potential as an Alternative to Dacarbazine. Cancer Res. 1987, 47, 5846–5852. [Google Scholar]
- Denny, B.J.; Wheelhouse, R.T.; Stevens, M.F.; Tsang, L.L.; Slack, J.A. NMR and Molecular Modeling Investigation of the Mechanism of Activation of the Antitumor Drug Temozolomide and its Interaction with DNA. Biochemistry 1994, 33, 9045–9051. [Google Scholar]
- Fukushima, T.; Takeshima, H.; Kataoka, H. Anti-glioma Therapy with Temozolomide and Status of the DNA-repair Gene MGMT. Anticancer Res. 2009, 29, 4845–4854. [Google Scholar]
- Zustovich, F.; Lombardi, G.; Della Puppa, A.; Rotilio, A.; Scienza, R.; Pastorelli, D. A Phase II Study of Cisplatin and Temozolomide in Heavily Pre-treated Patients with Temozolomide-refractory High-grade Malignant Glioma. Anticancer Res. 2009, 29, 4275–4279. [Google Scholar]
- Casey, D.A.; Wexler, L.H.; Merchant, M.S.; Chou, A.J.; Merola, P.R.; Price, A.P.; Meyers, P.A. Irinotecan and Temozolomide for Ewing Sarcoma: the Memorial Sloan-Kettering Experience. Pediatr. Blood Cancer 2009, 53, 1029–1034. [Google Scholar] [CrossRef]
- Lunt, E.; Newton, C.G.; Smith, C.; Stevens, G.P.; Stevens, M.F.G.; Straw, C.G.; Walsh, R.J.A.; Warren, P.J.; Fizames, C.; Lavelle, F.; Langdon, S.P.; Vickers, L.M. Antitumor Imidazotetrazines. 14. Synthesis and Antitumor Activity of 6- and 8-Substituted imidazo[5,1-d]-1,2,3,5-tetrazinones and 8-Substituted pyrazolo[5,1-d]-1,2,3,5-tetrazinones. J. Med. Chem. 1987, 30, 357–366. [Google Scholar] [CrossRef]
- Clark, A.S.; Deans, B.; Stevens, M.F.; Tisdale, M.J.; Wheelhouse, R.T.; Denny, B.J.; Hartley, J.A. Antitumor Imidazotetrazines. 32. Synthesis of Novel Imidazotetrazinones and Related Bicyclic Heterocycles to Probe the Mode of Action of the Antitumor Drug Temozolomide. J. Med. Chem. 1995, 38, 1493–1504. [Google Scholar] [CrossRef]
- Wang, Y.F.; Stevens, M.F.; Chan, T.M.; Dibenedetto, D.; Ding, Z.X.; Gala, D.; Hou, D.; Kugelman, M.; Leong, W.; Kuo, S.C.; Mas, J.L.; Schumacher, D.P.; Shutts, B.P.; Smith, L.; Zhan, Z.Y.J.; Thomson, W.T. Antitumor Imidazotetrazines. 35. New Synthetic Routes to the Antitumor Drug Temozolomide. J. Org. Chem. 1997, 62, 7288–7294. [Google Scholar]
- Hartman, W.W. β-Diethylaminoethyl Alcohol (Ethanol, 2-diethylamino). Org. Syn. Coll. 1955, II, 193–184. [Google Scholar]
- Buckingham, J. Dictionary of Organic Compounds, 6th ed; Chapman & Hall: New York, NY, USA, 1996; Volume 6, p. 5423. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, D.; Yang, J.-G.; Cheng, J.; Zhao, L.-X. Synthesis and Antitumor Activity of 3-Methyl-4-oxo-3,4-dihydroimidazo [5,1-d][1,2,3,5]tetrazine-8-carboxylates and -carboxamides. Molecules 2010, 15, 9427-9436. https://doi.org/10.3390/molecules15129427
Liu D, Yang J-G, Cheng J, Zhao L-X. Synthesis and Antitumor Activity of 3-Methyl-4-oxo-3,4-dihydroimidazo [5,1-d][1,2,3,5]tetrazine-8-carboxylates and -carboxamides. Molecules. 2010; 15(12):9427-9436. https://doi.org/10.3390/molecules15129427
Chicago/Turabian StyleLiu, Dan, Jian-Guo Yang, Jie Cheng, and Lin-Xiang Zhao. 2010. "Synthesis and Antitumor Activity of 3-Methyl-4-oxo-3,4-dihydroimidazo [5,1-d][1,2,3,5]tetrazine-8-carboxylates and -carboxamides" Molecules 15, no. 12: 9427-9436. https://doi.org/10.3390/molecules15129427