A New Triterpenoid Saponin from Pulsatilla cernua
Abstract
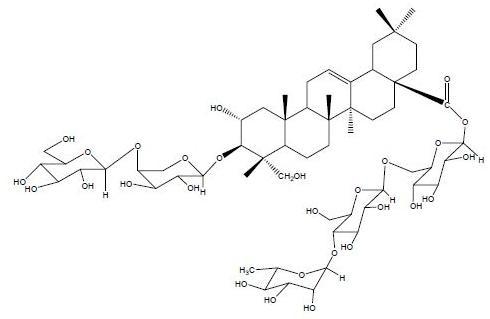
:1. Introduction
2. Results and Discussion

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of 1
3.5. Characterization of Compound 1
4. Conclusions
Acknowledgements
References
- Zhang, Q.W.; Ye, W.C.; Che, C.T.; Zhao, S.X. Triterpene saponins from pulsatilla cernua. Acta Pharm. Sin. 2000, 35, 756–759. [Google Scholar]
- Xu, T.H.; Xu, Y.J.; Han, D.; Zhao, H.F.; Xie, S.X.; Xu, D.M. Triterpenoid saponins from Pulsatilla cernua (Thunb.) Bercht. et Opiz. J. Integr. Plant Biol. 2007, 49, 202–206. [Google Scholar] [CrossRef]
- Xu, T.H.; Xu, Y.J.; Li, H.X.; Han, D.; Zhao, H.F.; Xie, S.X.; Li, Y.; Niu, J.Z.; Si, Y.S.; Xu, D.M. Two new triterpenoid saponins from Pulsatilla cernua (Thunb.) Bercht. et Opiz. J. Asia Nat. Prod. Res. 2007, 9, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, M.; Shirasuna, K.; Hirai, Y.; Shingu, K.; Isoda, S.; Shoji, J.; Ida, Y.; Shimizu, T. Triterpenoid Saponins of Acanthopanax nipponicus leaves. J. Nat. Prod. 1999, 62, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.B.; Kundu, A.P. 13C NMR spectra of pentacyclic triterpenoids-a compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Bhandari, P.; Gray, A.; Rastogi, R.P. Triterpenoid saponins from Caltha palustris. Planta Med. 1987, 53, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S. Saponins from the roots of Pulsatilla koreana. Arch. Pharm. Res. 1989, 12, 42–47. [Google Scholar] [CrossRef]
- Kawai, H.; Kuroyanagi, M.; Umehara, K.; Ueno, A.; Satake, M. Studies on the saponins of Lonicera japonica Thunb. Chem. Pharm. Bull. 1988, 36, 4769–4775. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.R.; Oinaka, T.; Kasai, R.; Tanaka, O. Saponins from Leaves of Kalopanax pictum var. maximowiczii, a Korean Medicinal Plant. Chem. Pharm. Bull. 1989, 37, 2234–2235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Wang, D.Z.; Wu, S.G.; Yang, C.R. Triterpenoid saponins from pulsatilla campanella. Phytochemistry 1990, 29, 595–599. [Google Scholar] [CrossRef]
- Grishkovets, V.I.; Sobolev, E.A.; Shashkov, A.S.; Chirva, V.Y. Triterpene glycosides of Fatsia japonica. I. Isolation and structure of glycosides from Fatsia japonica seeds. Chem. Nat. Compd. 2000, 36, 166–169. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Jain, D.C.; Gupta, R.K.; Thakur, R.S. Carbon-13 NMR spectroscopy of steroidal sapogenins and steroidal saponins. Phytochemistry 1985, 24, 2479–2496. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| No. | δc | δH (J, Hz) | No. | δc | δH (J, Hz) |
|---|---|---|---|---|---|
| 1 | 44.4 | C-3 | |||
| 2 | 71.0 | 4.20 | Ara-1 | 106.8 | 4.91 (d, 7.0 Hz) |
| 3 | 83.0 | 4.24 | 2 | 73.7 | 4.68 |
| 4 | 42.4 | 3 | 71.4 | 4.27 | |
| 5 | 47.9 | 4 | 80.4 | 4.80 | |
| 6 | 18.1 | 5 | 65.4 | 4.48, 4.39 | |
| 7 | 33.0 | Glc-1 | 107.3 | 5.22 (d, 8.0) | |
| 8 | 40.2 | 2 | 76.0 | 3.92 | |
| 9 | 48.7 | 3 | 78.9 | 4.15 | |
| 10 | 37.2 | 4 | 71.0 | 4.16 | |
| 11 | 23.8 | 5 | 78.6 | 3.89 | |
| 12 | 123.4 | 5.41 (t-like) | 6 | 62.6. | 4.29,4.45 (d, 7.6) |
| 13 | 144.2 | C-28 | |||
| 14 | 42.9 | Glc-1′ | 95.8 | 6.21 (d, 8.0) | |
| 15 | 28.4 | 2′ | 74.2 | 4.10 | |
| 16 | 23.5 | 3′ | 78.2 | 4.19 | |
| 17 | 46.3 | 4′ | 70.4 | 4.25 | |
| 18 | 41.8 | 3.15 (dd, 3.0, 12.5) | 5′ | 78.3 | 4.08 |
| 19 | 47.2 | 6′ | 69.4 | 4.29,4.62 | |
| 20 | 30.9 | Glc-1′′ | 105.0 | 4.91 (d, 7.9) | |
| 21 | 34.1 | 2′′ | 75.5 | 3.89 | |
| 22 | 32.7 | 3′′ | 76.7 | 4.11 | |
| 23 | 65.4 | 3.67 (d, 10.0) 4.54 | 4′′ | 78.9 | 4.34 |
| 24 | 15.1 | 1.00 (s) | 5′′ | 77.3 | 3.81 |
| 25 | 17.5 | 1.08 (s) | 6′′ | 61.4 | 4.06, 4.21 |
| 26 | 17.8 | 1.14 (s) | Rha-1 | 102.9 | 5.82 (br, s) |
| 27 | 26.7 | 1.18 (s) | 2 | 72.7 | 4.61 |
| 28 | 176.6 | 3 | 72.9 | 4.50 | |
| 29 | 33.2 | 0.85 (s) | 4 | 74.0 | 4.27 |
| 30 | 24.1 | 0.86 (s) | 5 | 70.9 | 4.85 |
| 6 | 18.7 | 1.71 (d, 6.0) |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, Y.; Bai, L.; Liu, Y.; Liu, Y.; Xu, T.; Xie, S.; Si, Y.; Zhou, H.; Liu, T.; Xu, D. A New Triterpenoid Saponin from Pulsatilla cernua . Molecules 2010, 15, 1891-1897. https://doi.org/10.3390/molecules15031891
Xu Y, Bai L, Liu Y, Liu Y, Xu T, Xie S, Si Y, Zhou H, Liu T, Xu D. A New Triterpenoid Saponin from Pulsatilla cernua . Molecules. 2010; 15(3):1891-1897. https://doi.org/10.3390/molecules15031891
Chicago/Turabian StyleXu, Yajuan, Lu Bai, Yonghong Liu, Yue Liu, Tunhai Xu, Shengxu Xie, Yunshan Si, Haiou Zhou, Tonghua Liu, and Dongming Xu. 2010. "A New Triterpenoid Saponin from Pulsatilla cernua " Molecules 15, no. 3: 1891-1897. https://doi.org/10.3390/molecules15031891