Dendrimers Containing Ferrocene and Porphyrin Moieties: Synthesis and Cubic Non-Linear Optical Behavior
Abstract
:1. Introduction
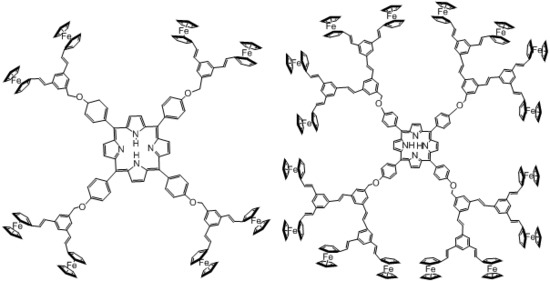
2. Results and Discussion




2.1. Linear and third order non-linear optical characterization


| Sample (50 wt. % into PS) | α × 105 (cm-1)a) | (× 10-12 esu)b) |
|---|---|---|
| 12 | 7.1 | 3.1 |
| 13 | 11.4 | 8.3 |
2.2. Crystal structure determination
| Empirical formula | C31 H27 Cl Fe2 |
|---|---|
| Formula weight | 546.68 |
| Temperature | 298(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Orthorhombic |
| Space group | Pnma |
| Unit cell dimensions | a = 8.8302(7) Å |
| b = 30.601(2) Å | |
| c = 9.349(1) Å | |
| Volume | 2526.2(4) Å3 |
| Z | 4 |
| Density (calculated) | 1.437 Mg/m3 |
| Absorption coefficient | 1.272 mm-1 |
| F(000) | 1128 |
| Crystal size / shape / color | 0.26 × 0.24 × 0.09 mm / Prism/ Red |
| Theta range for data collection | 2.28 to 25.36°. |
| Index ranges | -10<= h <=10, -36<= k <=36, -11<= l <=11 |
| Reflections collected | 19597 |
| Independent reflections | 2362 [R(int) = 0.0404] |
| Completeness to theta = 25.36° | 99.8 % |
| Absorption correction | Integration |
| Max. and min. transmission | 0.8941 and 0.7334 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 2362/60/179 |
| Goodness-of-fit on F2 | 1.090 |
| Final R indices [I>2sigma(I)] | R1 = 0.0465, wR2 = 0.1059 |
| R indices (all data) | R1 = 0.0591, wR2 = 0.1130 |
| Largest diff. peak and hole | 0.429 and -0.310 e. Å-3 |
3. Experimental
3.1. General
3.2. Synthesis of dendrons and dendrimers
3.3. THG Maker fringe measurements

4. Conclusions
Acknowledgements
- Sample Availability: Not available.
References and Notes
- Tomalia, D.A.; Dupont Durst, H. Genealogically directed synthesis: Starburst/cascade dendrimers and hyperbranched structures. Top. Curr. Chem. 1993, 165, 193–313. [Google Scholar]
- Newkome, G.R.; Moorefield, C.N. Comprehensive Supramolecular Chemistry; Pergamon: Oxford, UK, 1996; Volume 10, pp. 777–832. [Google Scholar]
- Fréchet, J.M.J. Functional polymers and dendrimers: Reactivity, molecular architecture, and interfacial energy. Science 1994, 263, 1710–1715. [Google Scholar]
- van Genderen, M.H.P.; Meijer, E.W. Supramolecular Technology; Wiley: New York, NY, USA, 1999; pp. 47–88, Chapter 2. [Google Scholar]
- Gorman, C. Metallodendrimers: Structural diversity and functional behavior. Adv. Mater. 1998, 10, 295–309. [Google Scholar] [CrossRef]
- Zeng, F.; Zimmerman, S.C. Dendrimers in supramolecular chemistry: From molecular recognition to self-assembly. Chem. Rev. 1997, 97, 1681–1712. [Google Scholar] [CrossRef]
- Fischer, M.; Vögtle, F. Dendrimers: From design to application - A progress report. Angew. Chem., Int. Ed. Engl. 1999, 38, 884–905. [Google Scholar] [CrossRef]
- Bhyrappa, P.; Young, J.K.; Moore, J.S.; Suslick, K.S. Shape selective epoxidation of alkenes by metalloporphyrin-dendrimers. J. Mol. Catal. A: Chem. 1996, 113, 109–116. [Google Scholar] [CrossRef]
- Bhyrappa, P.; Young, J.K.; Moore, J.S.; Suslick, K.S. Dendrimer-metalloporphyrins: Synthesis and catalysis. J. Am. Chem. Soc. 1996, 118, 5708–5711. [Google Scholar] [CrossRef]
- Hecht, S.; Fréchet, J.M.J. Singly and doubly oxidized phthalocyanine (pc) rings: [Cu(pc)(ReO4)] and [Cu(pc)(ReO4)2]. Angew. Chem., Int. Ed. 2001, 40, 244–246. [Google Scholar] [CrossRef]
- Piotti, M.E.; Rivera, F., Jr.; Bond, R.; Hawker, C.J.; Fréchet, J.M.J. Synthesis and catalytic activity of unimolecular dendritic reverse micelles with internal functional groups [13]. J. Am. Chem. Soc. 1999, 121, 9471–9472. [Google Scholar]
- Hollins, R.C. Materials for optical limiters. Curr. Opin. Solid State Mater. Sci. 1999, 4, 189–196. [Google Scholar] [CrossRef]
- Narayana, R.D. Excited state dynamics in porphyrins in relevance to third-order nonlinearity and optical limiting. Opt. Mater. 2003, 21, 45–49. [Google Scholar] [CrossRef]
- Papkovsky, D.B.; Ponomarev, G.V.; Tretnak, W.; O’Leary, P. Phosphorescent complexes of porphyrin ketones: optical properties and application to oxygen sensing. Anal. Chem. 1995, 67, 4112–4117. [Google Scholar] [CrossRef]
- Vinogradov, S.A.; Lo, L.-W.; Wilson, D.F. Dendritic polyglutamic porphyrins: Probing porphyrin protection by oxygen-dependent quenching of phosphorescence. Chem. Eur. J. 1999, 5, 1338–1347. [Google Scholar] [CrossRef]
- Rietveld, I.B.; Kim, E.; Vinogradov, S.A. Dendrimers with tetrabenzoporphyrin cores: Near infrared phosphors for in vivo oxygen imaging. Tetrahedron 2003, 59, 3821–3831. [Google Scholar] [CrossRef]
- Wasielewski, M.R. Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem. Rev. 1992, 92, 435–461. [Google Scholar] [CrossRef]
- Newkome, G.R.; He, E.; Moorefield, C.N. Suprasupermolecules with novel properties: Metallodendrimers. Chem. Rev. 1999, 99, 1689–1746. [Google Scholar] [CrossRef]
- Casado, C.M.; Cuadrado, I.; Morán, M.; Alonso, B.; García, B.; González, B.; Losada, J. Redox-active ferrocenyl dendrimers and polymers in solution and immobilised on electrode surfaces. Coord. Chem. Rev. 1999, 185, 53–79. [Google Scholar] [CrossRef]
- Peris, E. From long-chain conjugated oligomers to dendrimers: Synthesis and physical properties of phenyl-ethenyl-ferrocenyl containing one- and two-dimensional complexes. Coord. Chem. Rev. 2004, 248, 279–297. [Google Scholar]
- Alonso, B.; Cuadrado, I.; Moran, M. Losada, Organometallic silicon dendrimers. Chem. Soc. Chem. Commun. 1994, 2575–2577. [Google Scholar]
- Ruiz, J.; Pradet, C.; Varret, F.; Astruc, D. Molecular batteries: Synthesis and characterization of a dendritic 19-electron FeI complex that reduces C60 to its mono-anion. Chem. Commun. 2002, 1108–1109. [Google Scholar]
- Aranzaes, J.R.; Belin, C.; Astruc, D. Assembly of dendrimers with redox-active [{CpFe(μ3-CO)} 4] clusters at the periphery and their application to oxo-anion and adenosine-5′-triphosphate sensing. Angew. Chem. Int. Ed. 2005, 45, 132–136. [Google Scholar]
- Astruc, D.; Daniel, M.-C.; Ruiz, J. Dendrimers and gold nanoparticles as exo-receptors sensing biologically important anions. Chem. Commun. 2004, 2637–2649. [Google Scholar]
- Yoon, H.C.; Hong, M.-Y.; Kim, H.-S. Functionalization of a poly(amidoamine) dendrimer with ferrocenyls and its application to the construction of a reagentless enzyme electrode. Anal. Chem. 2000, 72, 4420–4427. [Google Scholar]
- Senge, M.O.; Fazekas, M.; Notaras, E.G.A.; Blau, W.J.; Zawadzka, M.; Locos, O.B.; Mhuircheartaigh, E.M. Nonlinear optical properties of porphyrins. Adv. Mater. 2007, 19, 2737–2774. [Google Scholar]
- Zhang, T.-G.; Zhao, Y.X.; Asselberghs, I.; Persoons, A.; Clays, K.; Therien, M.J. Design, synthesis, linear, and nonlinear optical properties of conjugated (porphinato)zinc(II)-based donor-acceptor chromophores featuring nitrothiophenyl and nitrooligothiophenyl electron-accepting moieties. J. Am.Chem. Soc. 2005, 127, 9710–9720. [Google Scholar]
- Goodson III, T.; Varnavski, O.; Wang, Y. Optical properties and applications of dendrimer-metal nanocomposites. Int. Rev. Phys. Chem. 2004, 23, 109–150. [Google Scholar] [CrossRef]
- Wang, W.; Sun, H.; Kaifer, E.A. Redox Active, Hybrid Dendrimers Containing Fréchet- and Newkome-Type Blocks. Org. Lett. 2007, 9, 2657–2660. [Google Scholar] [CrossRef]
- Ispasoiu, R.G.; Balogh, L.; Varnavski, O.P.; Tomalia, D.A.; Goodson III, T. Large optical limiting from novel metal - Dendrimer nanocomposite materials [1]. J. Am. Chem. Soc. 2000, 122, 11005–11006. [Google Scholar]
- Samoc, M.; Samoc, A.; Luther-Davies, B.; Humphrey, M.G.; Wong, M.S. Third-order optical nonlinearities of oligomers, dendrimers and polymers derived from solution Z-scan studies. Opt. Mater. 2003, 21, 485–487. [Google Scholar] [CrossRef]
- Powell, C.E.; Hurst, S.K.; Morrall, J.P.; Cifuentes, M.P.; Roberts, R.L.; Samoc, M.; Humphrey, M.G. Organometallic complexes for nonlinear optics. 39.1 syntheses and third-order nonlinear optical properties of first-generation peripherally metalated arylalkynyl dendrimers. Organometallics 2007, 26, 4456–4463. [Google Scholar]
- Wang, Y.; Xie, X.B.; Goodson III, T. Enhanced third-order nonlinear optical properties in dendrimer−metal nanocomposites. Nano Lett. 2005, 5, 2379–2384. [Google Scholar] [CrossRef]
- Luo, J.D.; Haller, M.; Ma, H.; Liu, S.; Kim, T.D.; Tian, Y.Q.; Chen, B.Q.; Jang, S.H.; Dalton, L.R.; Jen, A.K.J. Nanoscale architectural control and macromolecular engineering of nonlinear optical dendrimers and polymers for electro-optics. J. Phys. Chem. B. 2004, 108, 8523–8530. [Google Scholar]
- Varnavski, O.; Leanov, A.; Liu, L.; Takacs, J.; Goodson III, T. Large Nonlinear Refraction and Higher Order Nonlinear Optical Effects in a Novel Organic Dendrimer. J. Phys. Chem. B 2000, 104, 179–188. [Google Scholar] [CrossRef]
- West, R.; Wang, Y.; Goodson, T., III. Nonlinear absorption properties in novel gold nanostructured topologies. J. Phys. Chem. B. 2003, 107, 3419–3426. [Google Scholar] [CrossRef]
- Etienne, M.; Biney, A.; Walser, A.D.; Dorsinville, R.; Bauer, D.L.V.; Balogh-Nair, V. Third-order nonlinear optical properties of a cadmiun sulfide-dendrimer nanocomposite. Appl. Phys. Lett. 2005, 87, 181913–181915. [Google Scholar]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Lijanova, I.V.; Reyes-Valderrama, M.I.; Maldonado, J.L.; Ramos-Ortiz, G.; Klimova, T.; Martínez-García, M. Synthesis and cubic nonlinear optical behavior of phenyl and ferrocenyl-ended resorcinarene-based dendrimers. Tetrahedron 2008, 64, 4460–4467. [Google Scholar] [CrossRef]
- Kajzar, F.; Messier, J.; Rosilio, C. Nonlinear optical properties of thin films of polysilane. J. Appl. Phys. 1986, 60, 3040–3044. [Google Scholar] [CrossRef]
- Ramos-Ortiz, G.; Maldonado, J.L.; Meneses-Nava, M.A.; Barbosa-García, O.; Olmos-López, M.; Cha, M. Third-harmonic generation performance of organic polymer films doped with triphenylmethane derivative dyes. Opt. Mater. 2007, 29, 636–641. [Google Scholar] [CrossRef]
- Klimova, E.; Klimova, T.; Martínez-Mendoza, J.M.; Ortiz-Frade, L.; Maldonado, J.L.; Ramos-Ortiz, G.; Flores-Alamo, M.; Martínez-García, M. 5-Aryl-1-ferrocenylpenta-1,4-dien-3-ones: Synthesis, structures, electrochemistry and third-order nonlinear optical properties. Inorg. Chim. Acta 2009, 362, 2820–2827. [Google Scholar]
- Wang, X. H.; West, D. P.; McKeown, N. B.; King, T. A. Determining the cubic susceptibility χ(3) of films or glasses by the Maker fringe method: A representative study of spin-coated films of copper phthalocyanine derivation. J. Opt. Soc. Am. B 1998, 15, 1895–1903. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morales-Espinoza, E.G.; Sanchez-Montes, K.E.; Klimova, E.; Klimova, T.; Lijanova, I.V.; Maldonado, J.L.; Ramos-Ortíz, G.; Hernández-Ortega, S.; Martínez-García, M. Dendrimers Containing Ferrocene and Porphyrin Moieties: Synthesis and Cubic Non-Linear Optical Behavior. Molecules 2010, 15, 2564-2575. https://doi.org/10.3390/molecules15042564
Morales-Espinoza EG, Sanchez-Montes KE, Klimova E, Klimova T, Lijanova IV, Maldonado JL, Ramos-Ortíz G, Hernández-Ortega S, Martínez-García M. Dendrimers Containing Ferrocene and Porphyrin Moieties: Synthesis and Cubic Non-Linear Optical Behavior. Molecules. 2010; 15(4):2564-2575. https://doi.org/10.3390/molecules15042564
Chicago/Turabian StyleMorales-Espinoza, Eric G., Karla E. Sanchez-Montes, Elena Klimova, Tatiana Klimova, Irina V. Lijanova, José L. Maldonado, Gabriel Ramos-Ortíz, Simón Hernández-Ortega, and Marcos Martínez-García. 2010. "Dendrimers Containing Ferrocene and Porphyrin Moieties: Synthesis and Cubic Non-Linear Optical Behavior" Molecules 15, no. 4: 2564-2575. https://doi.org/10.3390/molecules15042564
APA StyleMorales-Espinoza, E. G., Sanchez-Montes, K. E., Klimova, E., Klimova, T., Lijanova, I. V., Maldonado, J. L., Ramos-Ortíz, G., Hernández-Ortega, S., & Martínez-García, M. (2010). Dendrimers Containing Ferrocene and Porphyrin Moieties: Synthesis and Cubic Non-Linear Optical Behavior. Molecules, 15(4), 2564-2575. https://doi.org/10.3390/molecules15042564