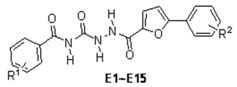
Synthesis and Bioactivity of N-Benzoyl-N'-[5-(2'-substituted phenyl)-2-furoyl] Semicarbazide Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological activity
3. Experimental
3.1. Materials and methods
3.2. General procedure for the preparation of key intermediates 2 and 4
3.3. General synthetic procedure for compounds E
3.4. X-ray structure determination of E2
3.5. Biological assays
4. Conclusions
Acknowledgements
References and Notes
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; San-Blas, G. Chitin synthesis as a target for antifungal drugs. Curr. Drug Targets Infect. Disord. 2003, 3, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Wellinga, K.; Mulder, R.; Dallen, J.V. Synthesis and laboratory evaluation of 1-(2,6-disubtituted benzoyl)-3-phenylureas, a new class of insecticides. I. 1-(2,5-Dichlorobenzoyl)-3- phenylureas. J. Agric. Food Chem. 1973, 21, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, H.; Silhacek, D.L. New Perspectives on the Mode of Action of Benzoylphenyl Urea Insecticides. In Insecticides with Novel Modes of Action: Mechanism and Application, 1st ed.; Ishaaya, I., Degheele, D., Eds.; Springer-Verlag: Berlin, Germany, 1998; pp. 92–105. [Google Scholar]
- Oberlander, H.; Silhacek, D.L. Mode of action of insect growth regulators in Lepidopteran tissue culture. Pestic. Sci. 1998, 54, 300–302. [Google Scholar] [CrossRef]
- Qian, X.H. Quantitative studies on structure-activity relationship of sulfonylurea and benzoylphenylurea type pesticides and their substituents’ bioisosterism using synthons’ activity contribution. J. Agric. Food Chem. 1999, 47, 4415–4418. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Kitahara, K.; Nishioka, T.; Iwamura, H.; Fujita, T. Quantitative structure- activity studies of benzoylphenylurea larvicides: I. Effect of substituents at aniline moiety against Chilo suppressalis walker. Pestic. Biochem. Physiol. 1984, 21, 309–325. [Google Scholar] [CrossRef]
- Tecle, B.; Ruzo, L.O.; Casida, J.E. Radiosynthesis of [benzoyl-3,4,5-3H]diflubenzuron by a route applicable to other high-potency Insect Growth Regulators. J. Agric. Food Chem. 1988, 36, 178–180. [Google Scholar] [CrossRef]
- Hashizume, B. Atabron 5E, a new IGR insecticide (chlorfluazuron). Jpn. Pestic. Inf. 1988, 58, 32–34. [Google Scholar]
- Sbragia, R.J.; Johnson, G.W.; Karr, L.L.; Edwards, J.M.; Schneider, B.M. Preparation of benzoylphenylurea insecticides to control cockroaches, ants, fleas, and termites. WO 9819542, 1998; [Chem. Abstr. 1998, 129, 13497].
- Yoon, C.; Yang, J.O.; Kang, S.H.; Kim, G.H. Insecticidal properties of bistrifluron against sycamore lace bug, Corythucha ciliata (Hemiptera:Tingidae). J. Pestic. Sci. 2008, 33, 44–50. [Google Scholar] [CrossRef]
- Grosscurt, A.C.; Van, H.R.; Wellinga, K. 1-Phenylcarbamoyl-2-pyrazolines, a new class of insecticides. 3. Synthesis and insecticidal properties of 3,4-diphenyl-1-phenylcarbamoyl-2-pyrazolines. J. Agric. Food Chem. 1979, 27, 406–409. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.M.; Huang, R.Q.; Mao, C.H.; Shang, J.; Bi, F.C. Synthesis and insecticidal evaluation of propesticides of benzoylphenylureas. J. Agric. Food Chem. 2005, 53, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Z.Q.; Wang, Q.M.; Shang, J.; Huang, R.Q.; Bi, F.C. Insecticidal benzoylphenylurea-S-carbamate: A new propesticide with two effects of both benzoylphenylureas and carbamates. J. Agric. Food Chem. 2007, 55, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.F.; Zheng, Y.L.; Chen, L.; Li, Y.Q.; Li, Q.S.; Song, H.B.; Huang, R.Q.; Bi, F.C.; Wang, Q.M. Design, synthesis and insecticidal activities of new N-benzoyl-N-phenyl-N- sulfenylureas. J. Agric. Food Chem. 2009, 57, 3661–3668. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Ling, Y.; Wang, D.Q.; Chen, F.H. The synthesis and biological activity of N-phenyl-N-(5-phenyl-2-furoyl) ureas. Chin. J. Synth. Chem. 2002, 10, 510–512. [Google Scholar]
- Yang, X.L.; Wang, D.Q.; Chen, F.H.; Ling, Y.; Zhang, Z.N.; Shang, Z.Z. Studies on the design, synthesis and biological activity of a novel type of acylurea compound. Chem. J. Chin. Univ. 1997, 18, 395–398. [Google Scholar]
- Yang, X.L.; Wang, D.Q.; Chen, F.H.; Ling, Y.; Zhang, Z.N. The synthesis and larvicidal activity of N-aroyl-N-(5-aryl-2-furoyl) ureas. Pestic. Sci. 1998, 52, 282–286. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.J.; Ling, Y.; Miao, H.J.; Shi, Y.X.; Yang, X.L. Synthesis and Fungicidal Activity of Aryl Carbamic Acid-5-aryl-2-furanmethyl Ester. J. Agric. Food Chem. 2010, 58, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.J.; Wang, R.B.; Komatsu, K.; Bonaz-Krause, P.; Zyrianov, Y.; McKenna, C.E.; Csipke, C.; Tokes, Z.A.; Lien, E.J. Synthesis, biological evaluation, and quantitative structure-activity relationship analysis of new Schiff bases of hydroxysemicarbazide as potential antitumor agents. J. Med. Chem. 2002, 45, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Nishat, N.; Ahamad, T.; Alshehri, S.M.; Parveen, S. Synthesis, characterization, and biocide properties of semicarbazide-formaldehyde resin and its polymer metal complexes. Eur. J. Med. Chem. 2010, 45, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Speziale, A.J.; Smith, L.R. New and convenient synthesis of acyl isocyanates. J. Org. Chem. 1962, 27, 3742–3743. [Google Scholar]
- Cui, Z.N.; Huang, J.; Li, Y.; Ling, Y.; Yang, X.L.; Chen, F.H. Synthesis and bioactivity of novel N,N′-diacylhydrazine derivatives containing furan(I). Chin. J. Chem. 2008, 26, 916–922. [Google Scholar] [CrossRef]
- Bruker (1998) SMART. Bruker AXS Inc.: Madsion, WI, USA, 1998.
- Sheldrick, G. M. (1997) SHELXS97 and SHELXL 97. University of Göttingen: Germany, 1997.
- Bruker (1999) SAINT and SHELXTL. Bruker AXS Inc.: Madison, WI, USA, 1999.
- Chen, N.C. Bioassay of Pesticides; Beijing Agricultural University Press: Beijing, China, 1991; pp. 161–162. [Google Scholar]
- Chen, N.C. Bioassay of Pesticides; Beijing Agricultural University Press: Beijing, China, 1991; p. 54. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; Gray-Goodrich, M.; Campbell,, H.; Mayo, J.; Boyd, M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Berkson, J. Application of the logistic function to bio-assay. J. Am. Statistical Assoc. 1944, 39, 357–365. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |



| Empirical formula | C19H12F3N3O4 |
| Formula weight | 403.32 |
| T | 173(2) K |
| Wavelength | 1.54178 Å |
| Crystal system | Triclinic |
| Space group | P1 |
| Unit cell dimensions | a = 6.8462(12) Å, α = 107.173(13)° b = 11.552(2) Å, β = 104.549(12)° c = 11.667(2) Å, γ = 93.363(13)° |
| Volume | 844.4(3) Å3 |
| Z | 2 |
| Dx | 1.586 Mg/m3 |
| Absorption coefficient | 1.171 mm-1 |
| F (0 0 0) | 412 |
| Crystal dimensions | 0.20 x 0.13 x 0.10 mm |
| θ range for data collection | 4.05 to 68.19 |
| Completeness to θ = 68.19 | 96.7 % |
| Limiting indices | -8≤h≤8, -13≤k≤13,-13≤l≤14 |
| Reflection collected/unique | 8794/2990 [R(int) = 0.0529] |
| Absorption correction | Numerical |
| Max. and min. transmission | 0.8919 and 0.7995 |
| Data/restraints/parameters | 2990 / 0 / 263 |
| Goodness-of-fit on F2 | 1.088 |
| Final R indices [I > 2σ (I)] | R1 = 0.0555, wR2 = 0.1218 |
| 2θmax | 136.38° with Cu Kα |
| (Δρ)max | 0.301 eÅ-3 |
| (Δρ)min | -0.318 eÅ-3 |
| Program system | SHELXS-97, SHELXL-97 |
| Structure determination | Direct method |
| Refinement | Full-matrix least-squares on F2 |
| CCDC No. | 769209 |
| Lengths | (Å) | Angles | (°) | Torsion angles | (°) |
| C(4)-C(7) | 1.461(4) | O(2)-C(11)-N(1) | 123.0(3) | C(11)-N(1)-N(2)-C(12) | -113.9(3) |
| C(8)-C(11) | 1.470(4) | O(2)-C(11)-C(8) | 123.4(3) | C(6)-C(1)-C(2)-C(3) | 2.0(5) |
| C(13)-C(14) | 1.504(4) | O(3)-C(12)-N(3) | 119.9(3) | F(1)-C(1)-C(2)-C(3) | -177.7(3) |
| O(2)-C(11) | 1.228(3) | O(3)-C(12)-N(2) | 124.9(3) | C(5)-C(4)-C(7)-O(1) | 176.3(2) |
| O(3)-C(12) | 1.224(3) | O(4)-C(13)-C(14) | 120.6(3) | C(7)-O(1)-C(8)-C(9) | 0.0(3) |
| O(4)-C(13) | 1.219(3) | O(4)-C(13)-N(3) | 124.2(3) | N(2)-N(1)-C(11)-O(2) | 4.9(4) |
| N(1)-C(11) | 1.363(3) | N(2)-C(12)-N(3) | 115.2(2) | N(1)-N(2)-C(12)-O(3) | 4.1(4) |
| N(2)-C(12) | 1.342(3) | N(3)-C(13)-C(14) | 115.2(3) | N(1)-N(2)-C(12)-N(3) | -176.2(2) |
| N(3)-C(13) | 1.369(3) | C(13)-N(3)-C(12) | 127.7(2) | C(12)-N(3)-C(13)-O(4) | 6.4(4) |
| N(3)-C(12) | 1.398(3) | C(12)-N(2)-N(1) | 119.9(2) | O(4)-C(13)-C(14)-C(19) | 122.7(3) |
| N(1)-N(2) | 1.387(3) | C(11)-N(1)-N(2) | 118.4(2) | C(17)-C(18)-C(19)-F(3) | 179.9(3) |
| Dihedral angles (°) | |
| Plane I and plane II | 4.2 |
| Plane I and plane III | 3.3 |
| Plane I and plane IV | 73.3 |
| Plane I and plane V | 17.5 |
| Plane II and plane III | 7.2 |
| Plane II and plane IV | 75.8 |
| Plane II and plane V | 19.9 |
| Plane III and plane IV | 70.1 |
| Plane III and plane V | 14.5 |
| plane IV and plane V | 55.9 |
| The mean deviation of the plane (Å) | |
| Plane I | 0.0099 |
| Plane II | 0.0009 |
| Plane III | 0.0144 |
| Plane IV | 0.0163 |
| Plane V | 0.0075 |
| Compd. | Inhibitory rate (%) | ||||
| Phytophthora Capsici | Botrytis Cinerea | Fusarium Oxysporum | Rhizoctonia Solanii | Corynespora Cassiicola | |
| E1 | 1.94 | 13.61 | 7.50 | 0.00 | 2.22 |
| E2 | 9.72 | 9.17 | 37.50 | 3.61 | 14.44 |
| E3 | 22.50 | 6.11 | 29.17 | 1.39 | 8.33 |
| E4 | 37.50 | 23.33 | 10.28 | 41.39 | 6.39 |
| E5 | 23.61 | 54.72 | 32.22 | 0.00 | 8.06 |
| E6 | 49.17 | 49.17 | 14.17 | 0.00 | 11.39 |
| E7 | 8.89 | 29.17 | 5.83 | 0.00 | 5.83 |
| E8 | 31.94 | 48.89 | 14.44 | 8.33 | 8.33 |
| E9 | 10.28 | 28.61 | 28.89 | 0.56 | 10.28 |
| E10 | 12.50 | 0.00 | 20.83 | 0.00 | 8.33 |
| E11 | 19.44 | 0.00 | 9.72 | 50.00 | 6.94 |
| E12 | 20.83 | 45.28 | 4.72 | 0.00 | 12.22 |
| E13 | 13.06 | 4.17 | 8.61 | 0.00 | 19.72 |
| E14 | 21.67 | 7.78 | 8.61 | 0.00 | 18.06 |
| E15 | 4.17 | 10.56 | 30.28 | 0.00 | 5.00 |
| DMF(control) | 4.17 | 5.56 | 22.78 | 13.06 | 14.72 |
| fungicidesa | 86.39 a | 69.72 b | 68.33 c | 76.67 d | 68.61 e |
| Compd. | Inhibitory rate (%) | ||
| Plutella xylostella L. | Aphis gossypii | Tetranchus urticae | |
| E1 | 15.0 | 20.0 | 0 |
| E2 | 32.0 | 20.0 | 0 |
| E3 | 33.0 | 29.4 | 0 |
| E4 | 30.0 | 17.1 | 0 |
| E5 | 25.0 | 23.1 | 0 |
| E6 | 20.0 | 12.9 | 0 |
| E7 | 14.0 | 27.3 | 0 |
| E8 | 24.0 | 32.7 | 0 |
| E9 | 0 | 18.5 | 0 |
| E10 | 95.0 | 15.4 | 0 |
| E11 | 45.0 | 23.2 | 0 |
| E12 | 16.0 | 19.2 | 0 |
| E13 | 10.0 | 38.1 | 0 |
| E14 | 0 | 24.5 | 0 |
| E15 | 85.0 | 14.8 | 0 |
| CK | 0 | 2.4 | 0 |
| Hexaflumuron | 100 | 22.6 | 100 |
| RH-5849 | 100 | 22.1 | 100 |
| Compd. | IC50 (mM) | |||
| HL-60 | Bel-7402 | BGC-823 | KB | |
| E1 | 1.56 | - | - | - |
| E2 | 1.26 | - | - | - |
| E3 | - | - | - | - |
| E4 | - | - | - | - |
| E5 | 0.13 | - | - | - |
| E6 | - | - | - | - |
| E7 | 11.99 | 5.39 | - | - |
| E8 | - | - | - | - |
| E9 | 5.39 | - | - | - |
| E10 | - | - | - | - |
| E11 | 0.44 | 11.02 | 1.42 | - |
| E12 | 2.93 | - | - | - |
| E13 | 0.18 | - | 59.46 | - |
| E14 | 2.63 | - | - | - |
| E15 | 0.14 | 0.36 | - | 0.09 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, Z.; Ling, Y.; Li, B.; Li, Y.; Rui, C.; Cui, J.; Shi, Y.; Yang, X. Synthesis and Bioactivity of N-Benzoyl-N'-[5-(2'-substituted phenyl)-2-furoyl] Semicarbazide Derivatives. Molecules 2010, 15, 4267-4282. https://doi.org/10.3390/molecules15064267
Cui Z, Ling Y, Li B, Li Y, Rui C, Cui J, Shi Y, Yang X. Synthesis and Bioactivity of N-Benzoyl-N'-[5-(2'-substituted phenyl)-2-furoyl] Semicarbazide Derivatives. Molecules. 2010; 15(6):4267-4282. https://doi.org/10.3390/molecules15064267
Chicago/Turabian StyleCui, Zining, Yun Ling, Baoju Li, Yongqiang Li, Changhui Rui, Jingrong Cui, Yanxia Shi, and Xinling Yang. 2010. "Synthesis and Bioactivity of N-Benzoyl-N'-[5-(2'-substituted phenyl)-2-furoyl] Semicarbazide Derivatives" Molecules 15, no. 6: 4267-4282. https://doi.org/10.3390/molecules15064267