HPLC and Anti-Inflammatory Studies of the Flavonoid Rich Chloroform Extract Fraction of Orthosiphon Stamineus Leaves
Abstract
:1. Introduction
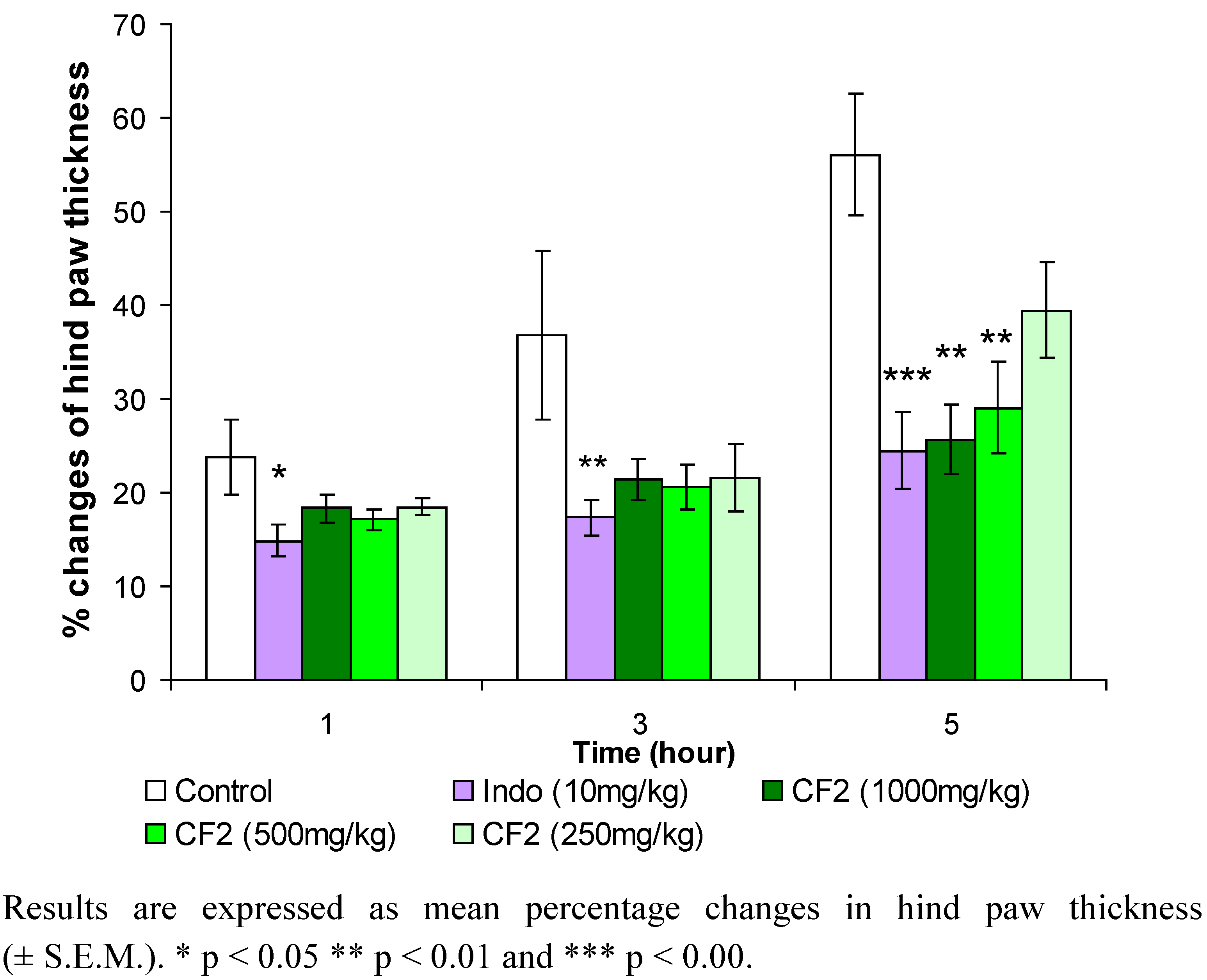
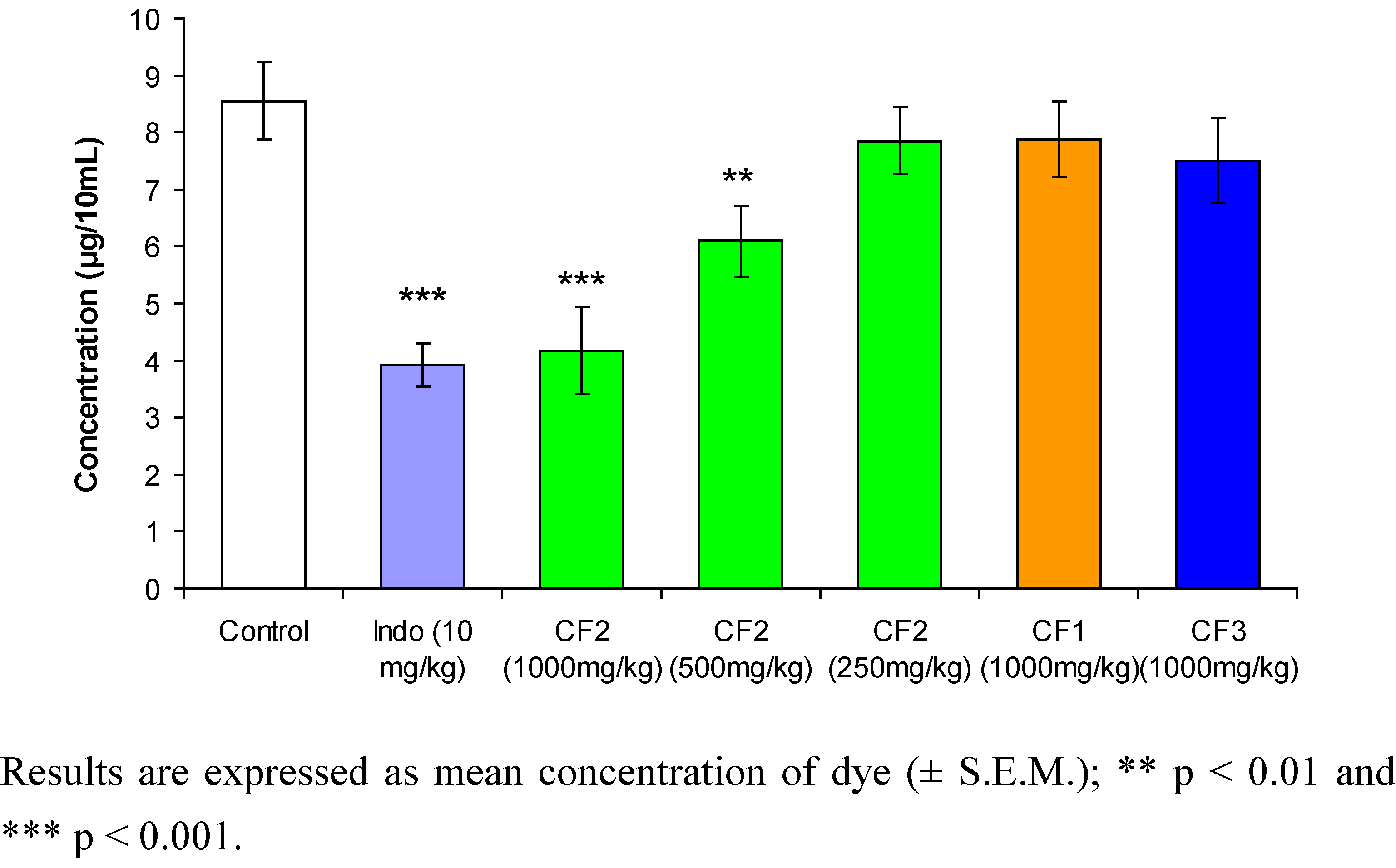
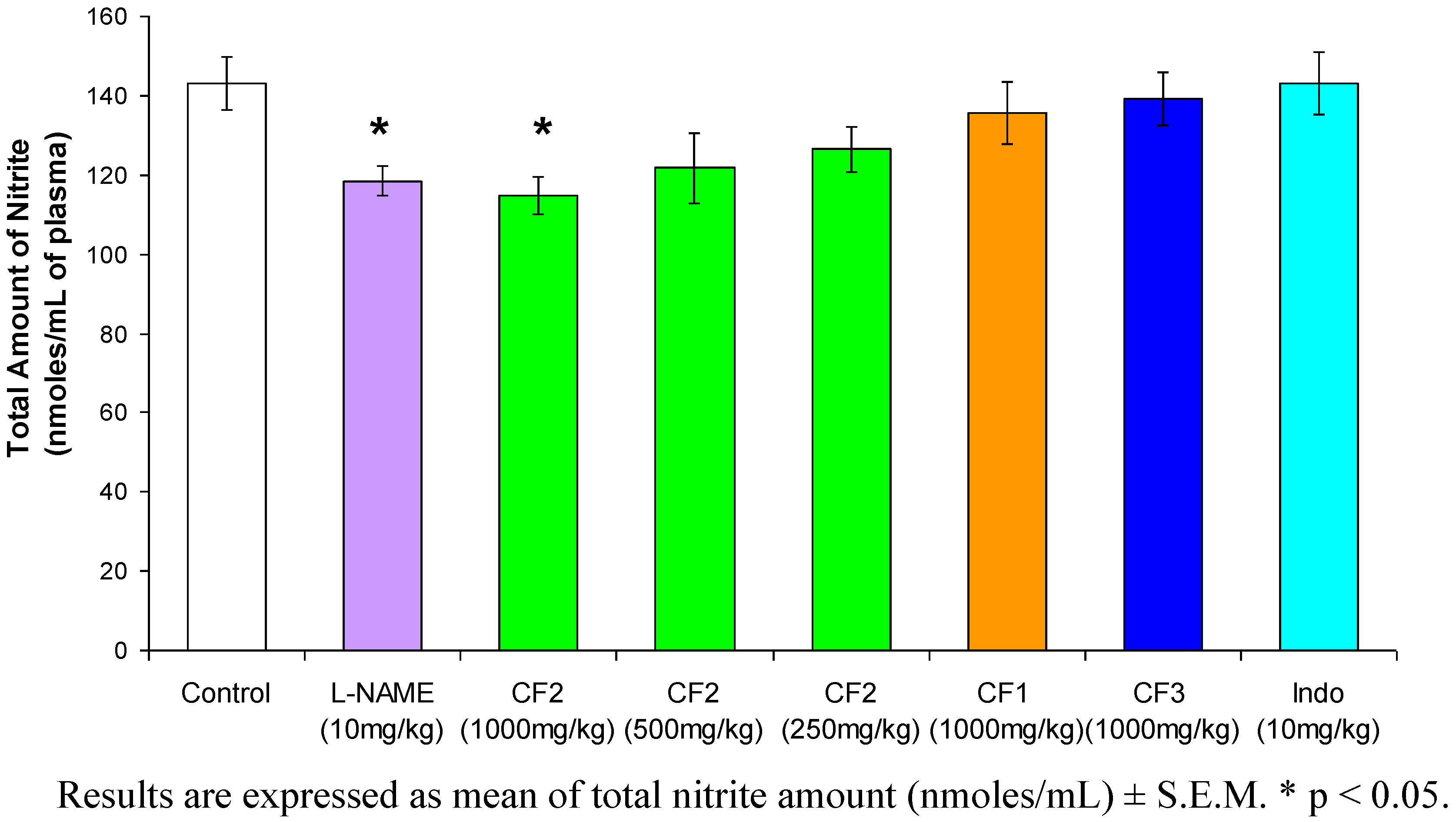
2. Results and Discussion



3. Experimental Section
3.1. Materials
3.2. Experimental animals
3.3. Plant material
3.4. Successive soxhlet-extraction and fractionation of chloroform extract
3.5. Carrageenan-induced hind paw edema test
3.6. Acetic acid-induced peritoneal capillary dye leakage test in mice
3.7. Carrageenan-induced nitric oxide
3.8. Griess assay
3.9. Assay for nitric oxide scavenging activity
3.10. HPLC analysis
3.10.1. Recovery studies
| Standard | Amount added (mg) | Recovery | |
|---|---|---|---|
| Mean (%) | R.S.D. (%) | ||
| Sinensetin | 0.17 | 101.5 | 0.83 |
| 0.13 | 97.73 | 4.07 | |
| 0.04 | 98.10 | 1.57 | |
| Eupatorin | 0.20 | 97.41 | 3.76 |
| 0.10 | 96.23 | 4.43 | |
| 0.05 | 102.0 | 2.30 | |
| TMF | 0.19 | 97.54 | 2.76 |
| 0.11 | 98.43 | 1.99 | |
| 0.06 | 99.23 | 3.89 | |
3.10.2. Precision studies
| Standard | Concentration (µg/mL) | R.S.D. (%) | |
|---|---|---|---|
| Intraday (n = 5) | Interday (n = 5) | ||
| Sinensetin | 100 | 2.54 | 3.67 |
| 50.0 | 4.35 | 3.10 | |
| 25.0 | 2.13 | 2.67 | |
| 12.5 | 1.27 | 2.17 | |
| 6.25 | 4.52 | 4.09 | |
| Eupatorin | 100 | 4.05 | 4.25 |
| 50.0 | 1.30 | 4.52 | |
| 25.0 | 1.56 | 4.15 | |
| 12.5 | 3.70 | 2.42 | |
| 6.25 | 1.69 | 3.10 | |
| TMF | 100 | 2.43 | 3.12 |
| 33.3 | 1.45 | 3.54 | |
| 11.1 | 3.21 | 3.98 | |
| 3.70 | 1.98 | 2.99 | |
| 1.23 | 3.67 | 3.45 | |
3.11. Isolation and structure determination of eupatorin and sinensetin

3.12. Experimental design and analysis of data
4. Conclusions
Acknowledgements
References
- Perry, L.M. Medicinal plants of East and Southeast Asia: Attributed Properties and Uses; The MIT Press: Cambridge, MA/London, UK, 1980. [Google Scholar]
- Olah, N.K.; Radu, L.; Mogosan, C.; Hanganu, D.; Gocan, S. Phytochemical and pharmacological studies on Orthosiphon stamineus Benth. (Lamiaceae) hydroalcoholic extracts. J. Pharm. Biomed. Anal. 2003, 33, 117–123. [Google Scholar] [CrossRef]
- Tezuka, Y.; Stampoulis, P.; Banskota, A.H.; Awale, S.; Tran, K.Q.; Saiki, I.; Kadota, S. Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. Chem. Pharm. Bull. 2000, 48, 1711–1719. [Google Scholar] [CrossRef]
- Hollman, P.C.; Katan, M.B. Dietary flavonoids: intake, health effects and bioavailability. Food. Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Lyckander, I.M.; Malterud, K.E. Lipophilic flavonoids from Orthosiphon spicatus prevent oxidative inactivation of 15-lipoxygenase. Prostag. Leukot. Essent. Fatty acids 1996, 54, 239–246. [Google Scholar] [CrossRef]
- Schut, G.A.; Zwaving, J.H. Pharmacological investigation of some lipophilic flavonoids from Orthosiphon aristatus. Fitoterapia 1993, LXIV 2, 99–102. [Google Scholar]
- Loon, Y.T.; Wong, J.W.; Yap, S.P.; Yuen, K.Y. Determination of flavonoids from Orthosiphon stamineus in plasma using a simple HPLC method with ultraviolet detection. J. Chromatogr. B 2005, 816, 161–166. [Google Scholar] [CrossRef]
- Awale, S.; Tezuka, Y.; Banskota, A.H.; Kouda, K.; Tun, K.M.; Kadota, S. Five novel highly oxygenated diterpenes of Orthosiphon stamineus from Myanmar. J. Nat. Prod. 2001, 64, 592–596. [Google Scholar] [CrossRef]
- Awale, A.; Tezuka, Y.; Banskota, A.H.; Kouda, K.; Tun, K.M.; Kadota, S. Four Highly oxygenated isopimarane-type diterpenes of Orthosiphon stamineus. Planta Med. 2002, 68, 286–288. [Google Scholar] [CrossRef]
- Awale, S.; Tezuka, Y.; Banskota, A.H.; Shimoji, S.; Taira, K.; Kadota, S. Norstaminane- and isopimarane-type diterpenes of Orthosiphon stamineus from Okinawa. Tetrahedron 2002, 58, 5503–5512. [Google Scholar] [CrossRef]
- Awale, A.; Tezuka, Y.; Kobayashi, M.; Ueda, J.; Kadota, S. Neoorthosiphonone A; a nitric oxide (NO) inhibitory diterpene with new carbon skeleton from Orthosiphon stamineus. Tetrahedron Lett. 2004, 45, 1359–1362. [Google Scholar] [CrossRef]
- Hussein, A.A.; Meyer, J.J.M.; Jimeno, M.L.; Rodríguez, B. Bioactive diterpenes from Orthosiphon labiatus and Salvia africana-lutea. J. Nat. Prod. 2007, 70, 293–295. [Google Scholar] [CrossRef]
- Masuda, T.; Masuda, K.; Shiragami, S.; Jitoe, A.; Nakatani, N. Orthosiphol A and B, novel diterpenoid inhibitors of TPA (12-O-tetradecanoylphorbol-13-acetate)-induced inflammation, from Orthosiphon stamineus. Tetrahedron 1992, 48, 6787–6792. [Google Scholar] [CrossRef]
- Yam, M.F.; Basir, R.; Asmawi, M.Z.; Ismail, Z. Antioixdant and hepatoprotective effects of Orthosiphon stamineus Benth standardized extract. Am. J. Chin. Med. 2007, 35, 117–128. [Google Scholar]
- Sriplang, K.; Adisakwattana, S.; Rungsipipat, A.; Yibchok-anun, S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats. J. Ethnopharm. 2007, 109, 510–514. [Google Scholar] [CrossRef]
- Abdullah, N.R.; Ismail, Z.; Ismail, Z. Acute toxicity of Orthosiphon stamineus Benth standardized extract in Sprague Dawley rats. Phytomedicine 2009, 16, 222–226. [Google Scholar] [CrossRef]
- Di Rosa, M. Biological properties of carrageenan. J. Pharmacy Pharmacol. 1972, 24, 89–102. [Google Scholar] [CrossRef]
- Ozaki, Y. Anti-inflammatory effects of Curcuma xanthorrhiza Roxb, and its active principle. Chem. Pharm. Bull. 1990, 38, 1045–1048. [Google Scholar]
- Di Rosa, M.; Giroud, J.P.; Willoughby, D.A. Study of the mediators of the acute inflammatory response induced in rats in different sites by carrageenin and turpentine. J. Path. 1971, 104, 15–29. [Google Scholar] [CrossRef]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Meth. Mol. Bio. 2003, 225, 115–121. [Google Scholar]
- Demello, S.V.B.; Novaes, G.S.; Laurindo, I.M.M.; Muscará, M.N.; Maciel, F.M.; Cossermelli, W. Nitric oxide synthase inhibitor influences prostaglandin and interleukin-1 production in experimental arthritis joints. Inflamm. Res. 1997, 46, 72–77. [Google Scholar] [CrossRef]
- Sautebin, L.; Ialenti, A.; Ianaro, A.; Di Rosa, M. Endogenous nitric oxide increases prostaglandin biosynthesis in carrageenin rat paw oedema. Eur. J. Pharmacol. 1995, 286, 219–222. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Zingarelli, B.; Costantino, G.; Szabó, A.; Salzman, A.L.; Caputi, A.P.; Szabó, C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 1997, 121, 1065–1074. [Google Scholar] [CrossRef]
- van Acker, S.A.B.E.; van den Berg, D.J.; Tromp, M.N.J.L.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radical Bio. Med. 1996, 20, 331–342. [Google Scholar]
- Sharp, J.T.; Gosney, I.; Rowley, A.G. Practical Organic Chemistry: A Student Handbook of Technique; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nus, G.V. Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drug. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar]
- Whittle, B.A. The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic analgesics. Br. J. Pharmacol. Chemother. 1964, 22, 246–253. [Google Scholar]
- Rockett, K.A.; Awburn, M.M.; Rockett, E.J.; Cowden, W.B.; Clark, I.A. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunology 1994, 16, 243–249. [Google Scholar] [CrossRef]
- Ravishankara, M.N.; Shrivastava, N.; Padh, H.; Rajani, M. Evaluation of antioxidant properties of root bark of Hemidesmus indicus R. Br. (Anantmul) Phytomedicine 2002, 9, 153–160. [Google Scholar]
- Szepesi, G. HPLC in Pharmaceutical Analysis; CRC Press Inc.: Boca Raton, FL, USA, 1990; Volume I. [Google Scholar]
- Sample Availability: Not available.
© 2010 by the authors;
Share and Cite
Yam, M.F.; Lim, V.; Salman, I.M.; Ameer, O.Z.; Ang, L.F.; Rosidah, N.; Abdulkarim, M.F.; Abdullah, G.Z.; Basir, R.; Sadikun, A.; et al. HPLC and Anti-Inflammatory Studies of the Flavonoid Rich Chloroform Extract Fraction of Orthosiphon Stamineus Leaves. Molecules 2010, 15, 4452-4466. https://doi.org/10.3390/molecules15064452
Yam MF, Lim V, Salman IM, Ameer OZ, Ang LF, Rosidah N, Abdulkarim MF, Abdullah GZ, Basir R, Sadikun A, et al. HPLC and Anti-Inflammatory Studies of the Flavonoid Rich Chloroform Extract Fraction of Orthosiphon Stamineus Leaves. Molecules. 2010; 15(6):4452-4466. https://doi.org/10.3390/molecules15064452
Chicago/Turabian StyleYam, Mun Fei, Vuanghao Lim, Ibrahim Muhammad Salman, Omar Ziad Ameer, Lee Fung Ang, Noersal Rosidah, Muthanna Fawzy Abdulkarim, Ghassan Zuhair Abdullah, Rusliza Basir, Amirin Sadikun, and et al. 2010. "HPLC and Anti-Inflammatory Studies of the Flavonoid Rich Chloroform Extract Fraction of Orthosiphon Stamineus Leaves" Molecules 15, no. 6: 4452-4466. https://doi.org/10.3390/molecules15064452





