Solid-Phase Synthesis of New Trp(Nps)-Containing Dipeptide Derivatives as TRPV1 Channel Blockers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis

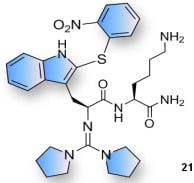
| Compound | R1 | Compound | R1 | R2 |
|---|---|---|---|---|
| 7, 17 |  | 12, 22 | – |  |
| 8, 18 |  | 13, 23 | – |  |
| 9, 19 |  | 14b, 24b |  |  |
| 10, 20 |  | 15a, 25a | – |  |
| 11, 21 |  | 15b, 25b |  |  |
| 16a, 26a | – |  | ||
| 16b, 26b |  |  |
2.2. Biological activity

3. Experimental
3.1. General
3.2. General procedure for the synthesis of Fmoc-Trp(Nps)-Lys(Boc or Alloc)-NH-PS (5, 6)
3.3. Typical procedure for Alloc removal
3.4. Reductive amination
3.5. Guanidylation reactions
3.5.1. N,N-di(Boc)-S-methylisothiourea as reagent
3.5.2. HBTU or HBPyU as reagent
3.5.3. Cleavage reactions
3.6. Biological activity
4. Conclusions
Acknowledgements
- Samples Availability: Please contact the authors.
References and Notes
- Levine, J.D.; Alessandri-Haber, N. TRP channels: Targets for the relief of pain. Biochim. Biophys. Acta 2007, 1772, 989–1003. [Google Scholar]
- Planells-Cases, R.; Garcìa-Sanz, N.; Morenilla-Palao, C.; Ferrer-Montiel, A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005, 451, 151–159. [Google Scholar] [CrossRef]
- Gunthorpe, M.J.; Chizh, B.A. Clinical development of TRPV1 antagonists: Targeting a pivotal point in the pain pathway. Drug Discov. Today 2009, 14, 56–67. [Google Scholar] [CrossRef]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef]
- García-Martínez, C.; Morenilla-Palao, C.; Planells-Cases, R.; Merino, J.M.; Ferrer-Montiel, A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J. Biol. Chem. 2000, 275, 32552–32558. [Google Scholar]
- Planells-Cases, R.; Aracil, A.; Merino, J.M.; Gallar, J.; Pérez-Payá, E.; Belmonte, C.; González-Ros, J.M.; Ferrer-Montiel, A. Arginine-rich peptides are blockers of VR-1 channels with analgesic activity. FEBS Lett. 2000, 481, 131–136. [Google Scholar] [CrossRef]
- García-López, M.T.; Herranz, R.; González-Muñiz, R.; Naranjo, J.R.; De Ceballos, M.; Del Río, J. Antinociceptive effects in rodents of the dipeptide Lys-Trp(Nps) and related compounds. Peptides 1986, 7, 39–43. [Google Scholar] [CrossRef]
- García-López, M.T.; González-Muñiz, R.; Molinero, M.T.; Del Río, J. Analgesic dipeptide derivatives. 4. Linear and cyclic analogues of the analgesic compounds arginyl-2-[(o-nitrophenyl)sulfenyl]tryptophan and lisyl-2-[(o-nitrophenyl)sulfenyl]tryptophan. J. Med. Chem. 1988, 31, 295–300. [Google Scholar] [CrossRef]
- Bonache, M.A.; García-Martínez, C.; García de Diego, L.; Carreno, C.; Pérez de Vega, M.J.; García-López, M.T.; Ferrer-Montiel, A.; González-Muñiz, R. Old molecule for new receptors: Trp(Nps) dipeptide derivatives as vanilloid TRPV1 channel blockers. ChemMedChem 2006, 1, 429–438. [Google Scholar] [CrossRef]
- Szardenings, A.K.; Burtoth, T.S.; Look, G.C.; Campbell, D.A. A reductive alkylation procedure applicable to both solution- and solid-phase syntheses of secondary amines. J. Org. Chem. 1996, 61, 6720–6722. [Google Scholar] [CrossRef]
- Shey, J.-Y.; Sun, C.-M. Soluble polymer supported synthesis of N,N-di(Boc)-protected guanidines. Synlett 1998, 1423–1425. [Google Scholar]
- Del Fresno, M.; El-Faham, A.; Carpino, L.A.; Royo, M.; Albericio F. Substituted guanidines: Introducing diversity in combinatorial chemistry. Org. Lett. 2000, 2, 3539–3542. [Google Scholar] [CrossRef]
- Farrera-Sinfreu, J.; Royo, M; Albericio, F. Undesired removal of the Fmoc group by the free ε-amino function of a lysine residue. Tetrahedron Lett. 2002, 43, 7813–7815. [Google Scholar] [CrossRef]
- García-Martínez, C.; Humet, M.; Planells-Cases, R.; Gomis, A.; Viana, F.; De La Peña, E.; Sáncez-Baeza, F.; Carbonell, T.; De Felipe, C.; Pérez-Payá, E.; Belmonte, C.; Messeguer, A.; Ferrer-Montiel, A. Attenuation of thermal nociception and hyperalgesia by VR1 blockers. Proc. Natl. Acad. Sci. USA 2002, 99, 2374–2379. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Vega, M.J.P.; García-López, M.T.; Zaccaro, L.; Royo, M.; Albericio, F.; Fernández-Carvajal, A.; Ferrer-Montiel, A.; González-Muñiz, R. Solid-Phase Synthesis of New Trp(Nps)-Containing Dipeptide Derivatives as TRPV1 Channel Blockers. Molecules 2010, 15, 4924-4933. https://doi.org/10.3390/molecules15074924
De Vega MJP, García-López MT, Zaccaro L, Royo M, Albericio F, Fernández-Carvajal A, Ferrer-Montiel A, González-Muñiz R. Solid-Phase Synthesis of New Trp(Nps)-Containing Dipeptide Derivatives as TRPV1 Channel Blockers. Molecules. 2010; 15(7):4924-4933. https://doi.org/10.3390/molecules15074924
Chicago/Turabian StyleDe Vega, Mª Jesús Pérez, Mª Teresa García-López, Laura Zaccaro, Miriam Royo, Fernando Albericio, Asia Fernández-Carvajal, Antonio Ferrer-Montiel, and Rosario González-Muñiz. 2010. "Solid-Phase Synthesis of New Trp(Nps)-Containing Dipeptide Derivatives as TRPV1 Channel Blockers" Molecules 15, no. 7: 4924-4933. https://doi.org/10.3390/molecules15074924
APA StyleDe Vega, M. J. P., García-López, M. T., Zaccaro, L., Royo, M., Albericio, F., Fernández-Carvajal, A., Ferrer-Montiel, A., & González-Muñiz, R. (2010). Solid-Phase Synthesis of New Trp(Nps)-Containing Dipeptide Derivatives as TRPV1 Channel Blockers. Molecules, 15(7), 4924-4933. https://doi.org/10.3390/molecules15074924