Casbane Diterpene as a Promising Natural Antimicrobial Agent against Biofilm-Associated Infections
Abstract
:1. Introduction
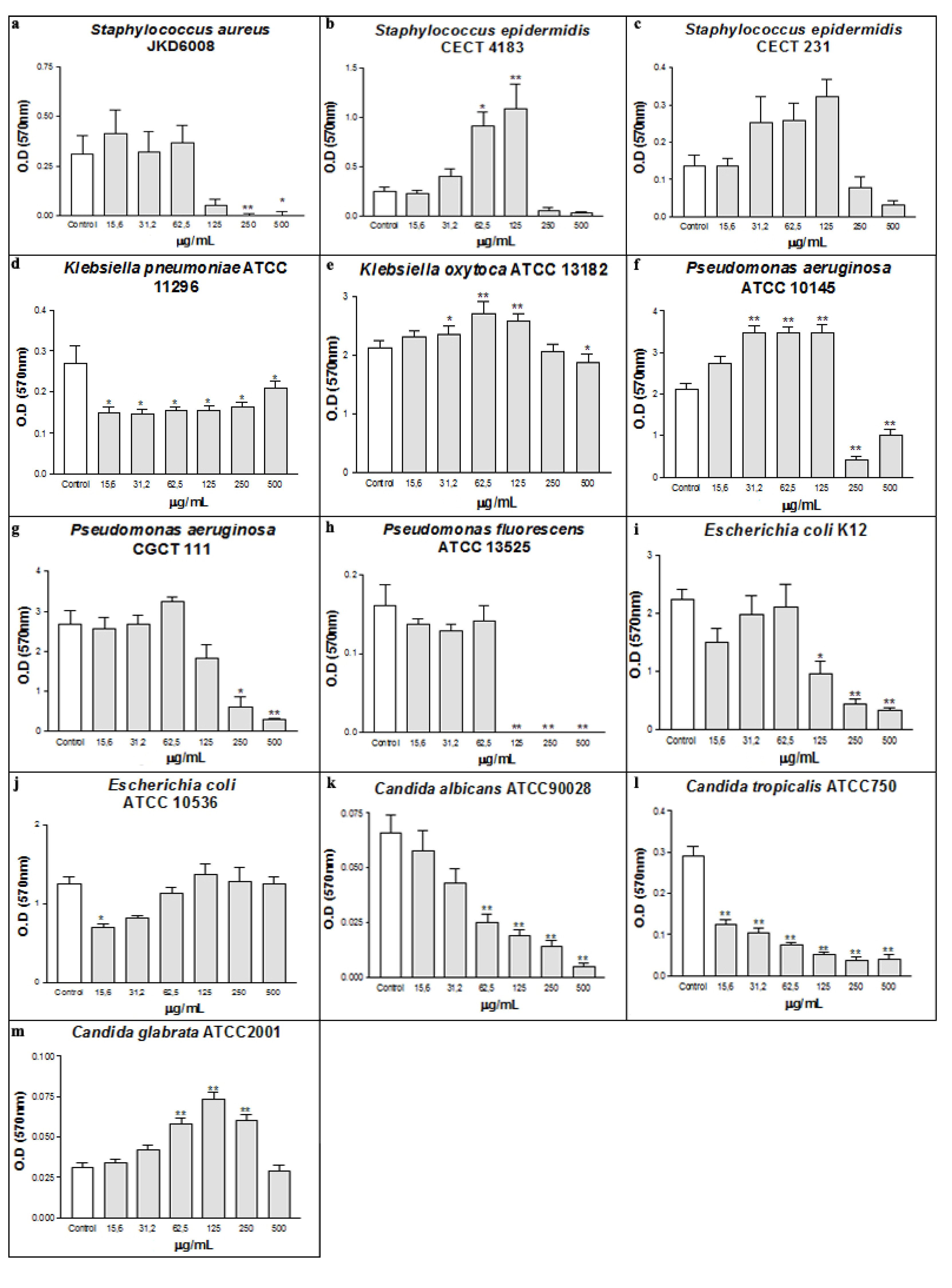
2. Results and Discussion

3. Experimental
3.1. Plant material
3.2. Extraction and isolation of casbane diterpene

3.3. Preparation of the CD stock solution
3.4. Microorganisms
3.5. Culture conditions
3.6. Antimicrobial assays
3.7. Antibiofilm activity
3.8. Statistical analysis
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References and Notes
- Rehm, B.H.A. Bacterial polymers: biosynthesis, modifications and applications. Appl. Ind. Microbiol. 2010, 8, 578–592. [Google Scholar]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.G.; Clegg, S. Role of MrkJ, a phosphodiesterase, in type 3 fimbrial expression and biofilm formation in Klebsiella pneumoniae. J. Bacteriol. 2010, 192, 3944–3950. [Google Scholar] [CrossRef]
- Kajiyama, S.; Tsurumoto, T.; Osaki, M.; Yanagihara, K.; Shindo, H. Quantitative analysis of Staphylococcus epidermidis biofilm on the surface of biomaterial. J. Orthopaed. Sci. 2009, 14, 769–767. [Google Scholar] [CrossRef]
- Bremer, P.J.; Fillery, S.; McQuillan, A.J. Laboratory scale Clean-In-Place (CIP) studies on the effectiveness of different caustic and acid wash steps on the removal of dairy biofilms. Int. J. Food Microbiol. 2006, 106, 254–262. [Google Scholar] [CrossRef]
- Hammond, A.M.S.; Dertien, J.M.S.; Colmer-Hamood, J.A.; Griswold, J.A.M.D.; Hamood, A.N. Serum Inhibits P. aeruginosa Biofilm Formation on Plastic Surfaces and Intravenous. J. Surg. Res. 2010, 159, 735–746. [Google Scholar] [CrossRef]
- Toney, J.H.; Koh, M.L. Inhibition of Xylella fastidiosa Biofilm Formation via Metal Chelators. J. Assoc. Lab. Automat. 2006, 11, 30–32. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef]
- Cateau, E.; Berjeaud, J.M.; Rodiera, M.H.; Christine, I. Fungal biofilm inhibition by a component naturally produced by Candida albicans yeasts growing as a biofilm. Int. J. Antimicrob. Agents 2008, 31, 166–170. [Google Scholar] [CrossRef]
- Wanga, X.; Yao, X.; Zhua, Z.; Tanga, T.; Daia, K.; Sadovskayac, I.; Flahautc, S.; Jabbouri, S. Effect of berberine on Staphylococcus epidermidis biofilm formation. Int. J. Antimicrob. Agents 2009, 34, 60–66. [Google Scholar] [CrossRef]
- Okuda, K.; Hanada, N.; Usui, Y.; Takeuchi, H.; Koba, H.; Nakao, R.; Watanabe, H.; Senpuku, H. Inhibition of Streptococcus mutans adherence and formation using analogues of the SspB peptide. Arch. Oral Biol. 2010, 55, 754–762. [Google Scholar] [CrossRef]
- Ndi, C.P.; Semple, S.J.; Griesser, H.J.; Pyke, S.M.; Barton, M.D. Antimicrobial Compounds from the Australian Desert Plant Eremophila neglecta. J. Nat. Prod. 2007, 70, 1439–1443. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Viano, Y.; Bonhomme, D.; Camps, M.; Briand, J.F.; Ortalo-Magné, A.; Blache, Y.; Piovetti, L.; Culioli, G. Diterpenoids from the Mediterranean brown alga Dictyota sp. evaluated as antifouling substances against a marine bacterial biofilm. J. Nat. Prod. 2009, 72, 1299–1304. [Google Scholar] [CrossRef]
- Behal, V. Nontraditional microbial bioactive metabolites. Folia Microbiol. 2001, 46, 363–370. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Murillo, R.M.; Jakupovic, J.; Rivera, J.; Castro, V.H. Diterpenes and other constituents from Croton draco (Euphorbiaceae). Rev. Biol. Trop. 2001, 49, 259–264. [Google Scholar]
- Araujo-Junior, V.T.; da Silva, M.S.; da Cunha, E.V.L.; Agra, M.D.; da Silva, R.N.; Barbosa, J.M.; Braz-Filho, R. Alkaloids and Diterpenes From Croton moritibensis. Pharm. Biol. 2004, 42, 62–67. [Google Scholar] [CrossRef]
- Peres, M.T.L.P.; Monache, F.D.; Cruz, A.B.; Pizzolatti, M.G.; Yunes, R.A.J. Chemical composition and antimicrobial activity of Croton urucurana Baillon (Euphorbiaceae). J.Ethnopharmacol. 1997, 56, 223–226. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Pinto, A.C.; Arruda, A.C.; Pamplona, S.G.S.R.; Vanderlinde, F.A.; Lapa, A.J.; Echevarria, A.; Grynberg, N.F.; Côlus, I.M.S.; Farias, R.A.F.; Costa, A.M.L.; Rao, V.S.N. Ethnopharmacology, phytochemistry and pharmacology: a successful combination in the study of Croton cajucara. J. Ethnopharmacol. 2000, 70, 41–55. [Google Scholar] [CrossRef]
- Graikou, K.; Aligiannis, N.; Skaltsounis, A.L.; Chinou, I.; Michel, S.; Tillequin, F.; Litaudon, M. New diterpenes from Croton insularis. J. Nat. Prod. 2004, 67, 685–688. [Google Scholar] [CrossRef]
- Peres, M.T.L.P.; Monache, F.D.; Pizzolatti, M.G.; Santos, A.R.S.; Beirith, A.; Calixto, J.B.; Yunes, R.A. Analgesic compounds of Croton urucurana Baillon. Pharmaco-chemical criteria used in their isolation. Phytother. Res. 1998, 12, 209–211. [Google Scholar] [CrossRef]
- Guadarrama, A.B.A.; Rios, M.Y. Three new sesquiterpenes from Croton arboreus. J. Nat. Prod. 2004, 67, 914–917. [Google Scholar] [CrossRef]
- McChesney, J.D.; Silveira, E.R. Ent-clerodanes of Croton sonderianus. Fitoterapia 1990, 61, 172–175. [Google Scholar]
- El Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry 2000, 53, 457–464. [Google Scholar]
- Barbosa, P.R.; Fascio, M.; Martins, D.; Guedes, M.L.S.; Roque, N.F. Triterpenes of Croton betulaster (Euphorbiaceae). Biochem. Syst. Ecol. 2003, 31, 307–308. [Google Scholar] [CrossRef]
- Giang, P.M.; Son, P.T.; Lee, J.H.; Lee, J.J.; Otsuka, H. Four ent-kaurane diterpenoids from Croton tonkinensis Gagnep. Chem. Pharm. Bull. 2004, 52, 879–882. [Google Scholar] [CrossRef]
- Santos, H.S.; Barros, F.W.A.; Albuquerque, M.R.J.R.; Bandeira, P.N.; Pessoa, C.; Braz-Filho, R.; Monte, F.J.Q.; Leal-Cardoso, J.H.; Lemos, T.L.G. Cytotoxic Diterpenoids from Croton argyrophylloides. J. Nat. Prod. 2009, 72, 1884–1887. [Google Scholar] [CrossRef]
- Santos, H.S.; Mesquita, F.M.R.; Lemos, T.L.G.; Monte, F.J.Q.; Braz-Filho, R. Diterpenos casbanos e acetofenonas de Croton nepetaefolius (Euphorbiaceae). Quím. Nova 2008, 31, 601–604. [Google Scholar] [CrossRef]
- Sarikahya, N.B.; Kirmizigül, S. Antimicrobial triterpenoid glycosides from Cephalaria scoparia. J. Nat. Prod. 2010, 73, 825–830. [Google Scholar] [CrossRef]
- Souza, A.B.; Martins, C.H.; Souza, M.G.; Furtado, N.A.; Heleno, V.C.; de Sousa, J.P.; Rocha, E.M.; Bastos, J.K.; Cunha, W.R.; Veneziani, R.C.; Ambrósio, S.R. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phytother. Res. 2010. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Roy, H. Tuning the properties of the bacterial membrane with aminoacylated phosphatidylglycerol. IUBMB Life 2009, 61, 940–953. [Google Scholar] [CrossRef]
- Priyadarshini, R.; de Pedro, M.A.; Young, K.D. Role of peptidoglycan amidases in the development and morphology of the division septum in Escherichia coli. J. Bacteriol. 2007, 189, 5334–5347. [Google Scholar] [CrossRef]
- Coenye, T.; Honraet, K.; Rigole, P.; Nadal, J.P.; Nelis, H.J. In vitro inhibition of Streptococcus mutans biofilm formation on hydroxyapatite by subinhibitory concentrations of anthraquinones. Antimicrob. Agents Chemother. 2007, 51, 1541–1544. [Google Scholar] [CrossRef]
- Xiang, W.; Song, Q.S.; Zhang, H.J.; Guo, S.P. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia 2008, 79, 501–504. [Google Scholar] [CrossRef]
- Silipo, A.; Molinaro, A. The diversity of the core oligosaccharide in lipopolysaccharides. Subcell. Biochem. 2010, 53, 69–99. [Google Scholar] [CrossRef]
- Xu, K.D.; McFeters, G.A.; Stewart, P.S. Biofilm resistance to antimicrobial agents. Microbiology 2000, 3, 547–549. [Google Scholar]
- Taweechaisupapong, S.; Singhara, S.; Lertsatitthanakorn, P.; Khunkitti, W. Antimicrobial effects of Boesenbergia pandurata and Piper sarmentosum leaf extracts on planktonic cells and biofilm of oral pathogens. Pakistan J. Pharm. Sci. 2010, 23, 224–231. [Google Scholar]
- Kuzma, L.; Rózalski, M.; Walencka, E. Rózalska, B.; Wysokinska, H. Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: Salvipisione as a potential anti-biofilm agent active against antibiotic resisrant Staphylococci. Phytomedicine 2007, 14, 31–35. [Google Scholar]
- Majtán, J.; Majtánová, L.; Xu, M.; Majtán, V. In vitro effect of subinhibitory concentrations of antibiotics on biofilm formation by clinical strains of Salmonella enterica serovar Typhimurium isolated in Slovakia. J. Appl. Microbiol. 2007, 104, 1294–1301. [Google Scholar]
- Lau, P.C.; Lindhout, T.; Beveridge, T.J.; Dutcher, J.R.; Lam, J.S. Differential lipopolysaccharide core capping leads to quantitative and correlated modifications of mechanical and structural properties in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2009, 191, 6618–6631. [Google Scholar] [CrossRef]
- Rocchetta, H.L.; Burrows, L.L.; Lam, J.S. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 1999, 63, 523–553. [Google Scholar]
- Singh, A.; Prasad, T.; Kapoor, K.; Mandal, A.; Roth, M.; Welti, R.; Prasad, R. Phospholipidome of Candida: Each Species of Candida Has Distinctive Phospholipid Molecular Species. OMICS A J. Integr. Biol. 2010, 14, 1–14. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Burton, E.; Yakandawala, N.; LoVetri, K.; Madhyastha, M.S. A microplate spectrofluorometric assay for bacterial biofilms. J. Ind. Microbiol. Biotechnol. 2007, 34, 1–4. [Google Scholar]
- Sample Availability: Contact the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carneiro, V.A.; Santos, H.S.d.; Arruda, F.V.S.; Bandeira, P.N.; Albuquerque, M.R.J.R.; Pereira, M.O.; Henriques, M.; Cavada, B.S.; Teixeira, E.H. Casbane Diterpene as a Promising Natural Antimicrobial Agent against Biofilm-Associated Infections. Molecules 2011, 16, 190-201. https://doi.org/10.3390/molecules16010190
Carneiro VA, Santos HSd, Arruda FVS, Bandeira PN, Albuquerque MRJR, Pereira MO, Henriques M, Cavada BS, Teixeira EH. Casbane Diterpene as a Promising Natural Antimicrobial Agent against Biofilm-Associated Infections. Molecules. 2011; 16(1):190-201. https://doi.org/10.3390/molecules16010190
Chicago/Turabian StyleCarneiro, Victor Alves, Hélcio Silva dos Santos, Francisco Vassiliepe Sousa Arruda, Paulo Nogueira Bandeira, Maria Rose Jane Ribeiro Albuquerque, Maria Olívia Pereira, Mariana Henriques, Benildo Sousa Cavada, and Edson Holanda Teixeira. 2011. "Casbane Diterpene as a Promising Natural Antimicrobial Agent against Biofilm-Associated Infections" Molecules 16, no. 1: 190-201. https://doi.org/10.3390/molecules16010190





