Functionalized 4-Hydroxy Coumarins: Novel Synthesis, Crystal Structure and DFT Calculations
Abstract
:1. Introduction
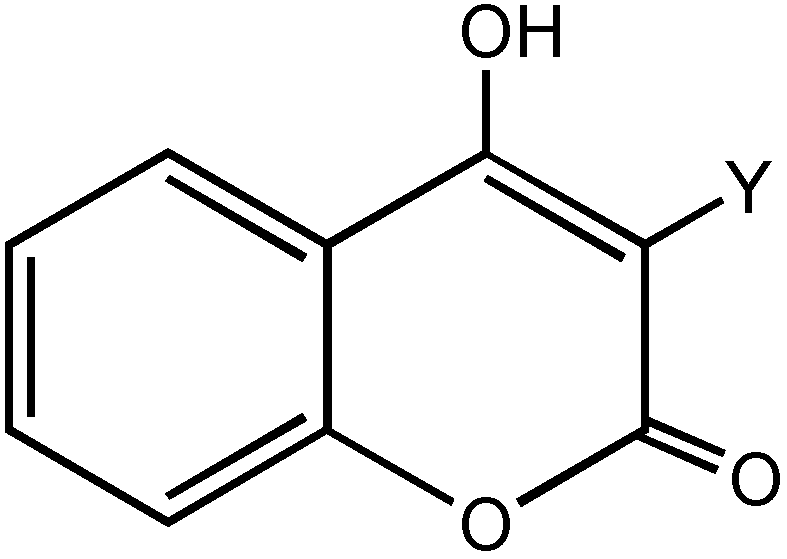

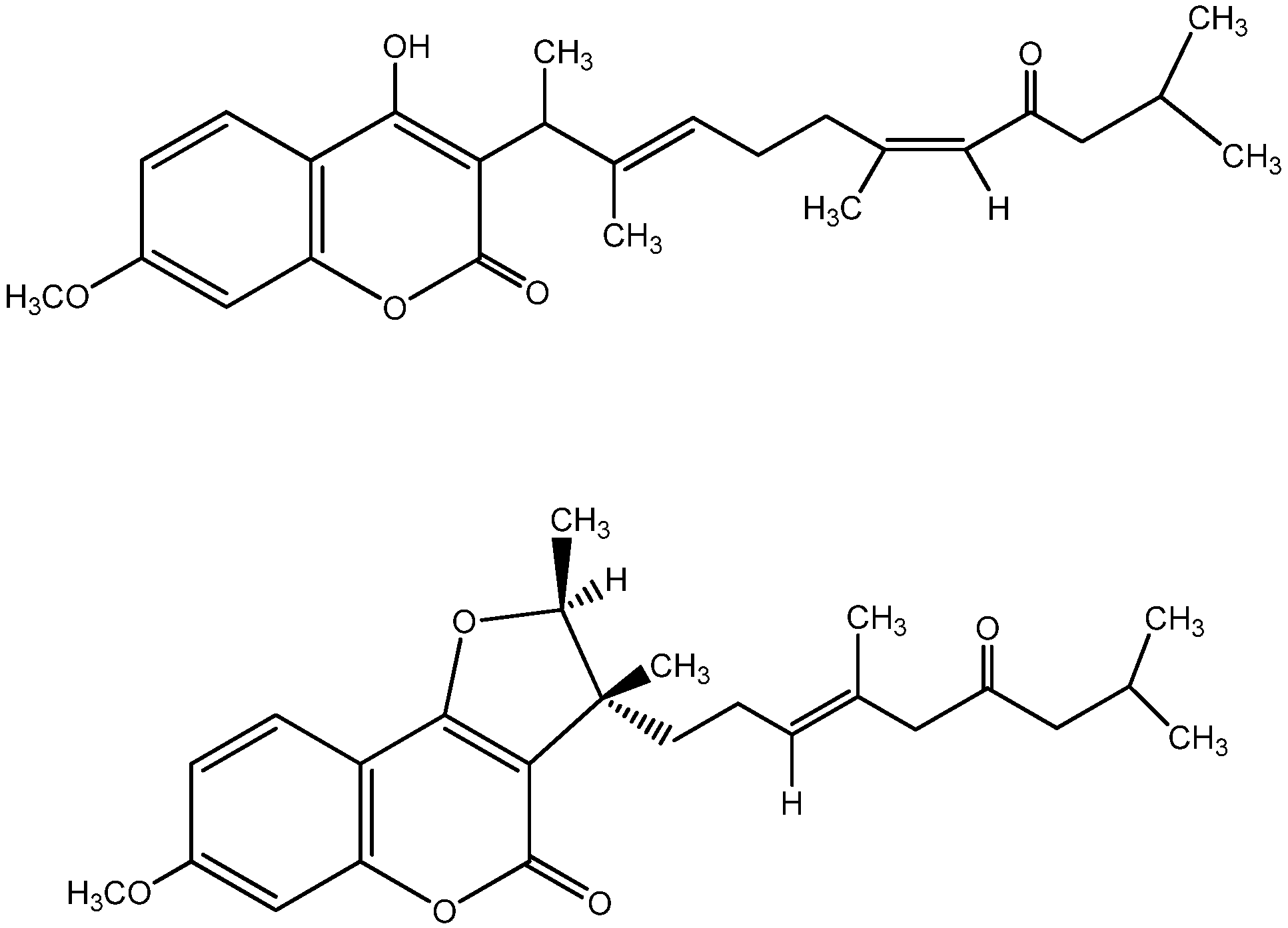
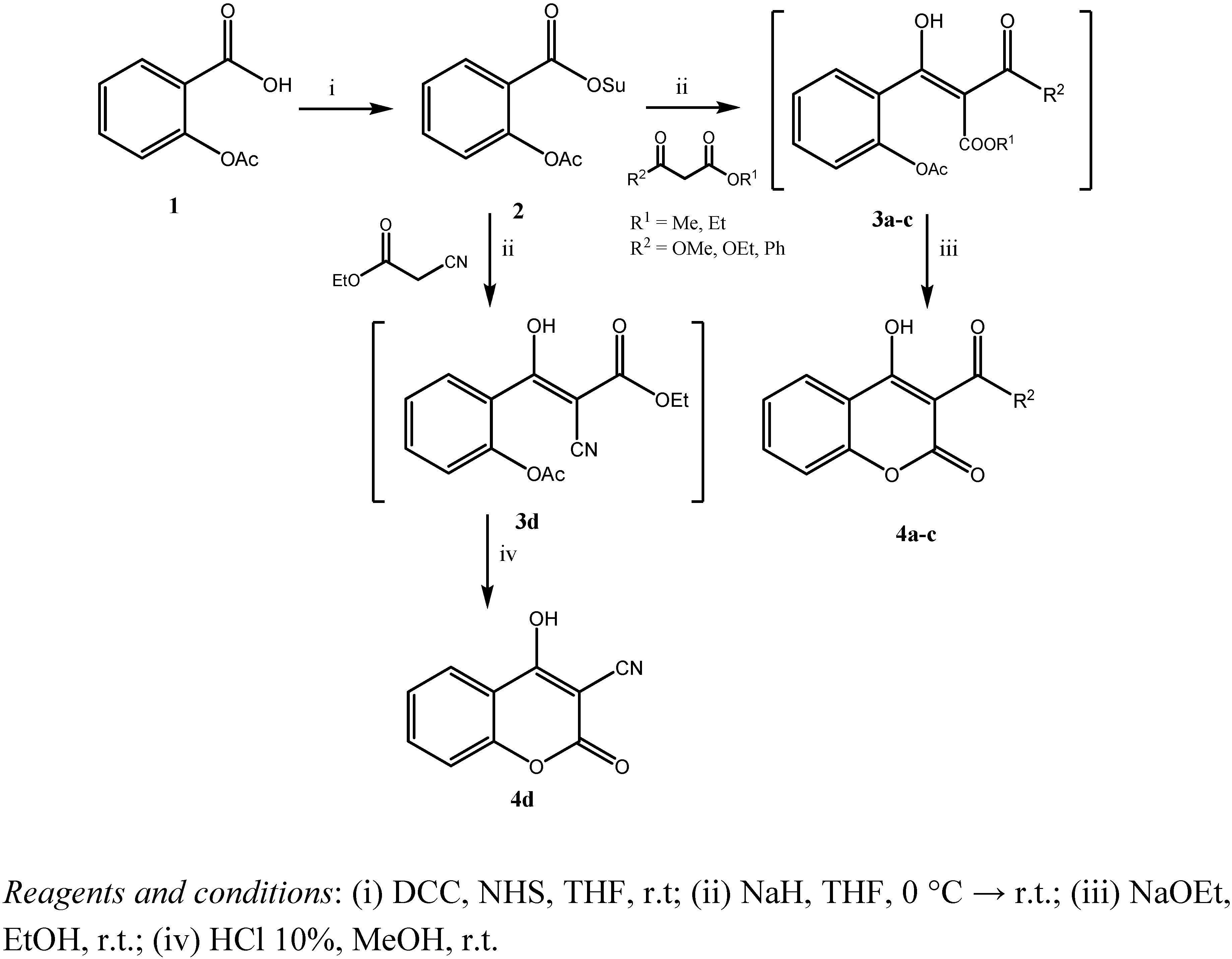
2. Results and Discussion
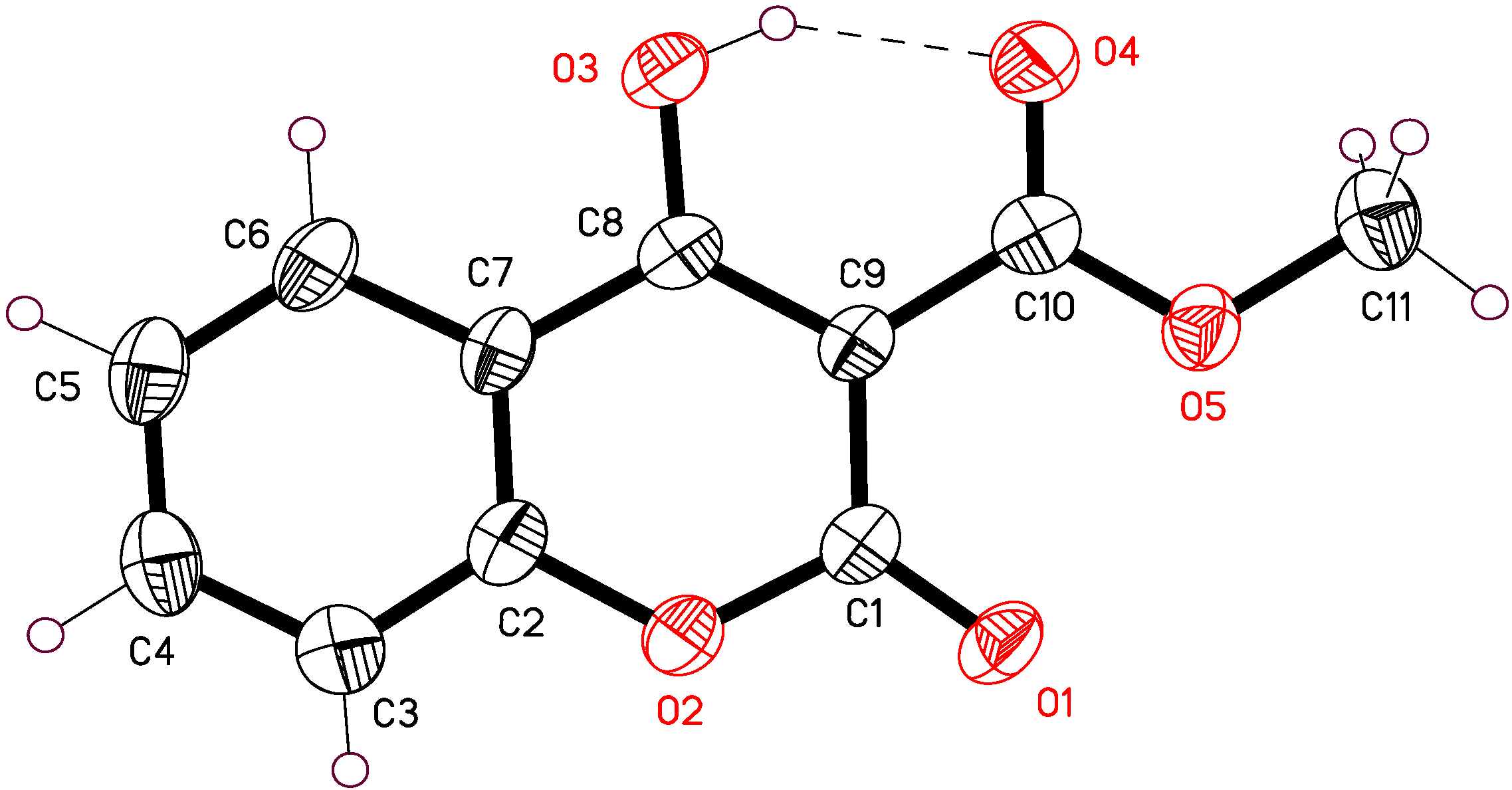
2.1. X-ray Crystallographic Analysis
| Empirical formula | C11H8O5 |
| Formula weight | 220.17 |
| Temperature | 150(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P2(1)/c |
| Unit cell dimensions | a = 3.802(3) Å |
| b = 21.945(15) Å; β= 90.097(10)°. | |
| c = 11.352(8) Å | |
| Volume | 947.1(11) Å3 |
| Z | 4 |
| Density (calculated) | 1.544 Mg/m3 |
| Absorption coefficient | 0.124 mm−1 |
| F(000) | 456 |
| Crystal size | 0.44 × 0.10 × 0.07 mm3 |
| Crystal description | colourless block |
| Theta range for data collection | 0.93 to 25.00°. |
| Index ranges | −4 ≤ h ≤ 4, −25 ≤ k ≤ 26, −13 ≤ l ≤ 13 |
| Reflections collected | 7326 |
| Independent reflections | 1686 [Rint = 0.0758] |
| Completeness to theta = 25.00° | 100.0% |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9914 and 0.9474 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 1686 / 0 / 149 |
| Goodness-of-fit on F2 | 1.028 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0662, wR2 = 0.1633 |
| R indices (all data) | R1 = 0.0984, wR2 = 0.1897 |
| Largest diff. peak and hole | 0.348 and -0.399 × 10−3 Å |
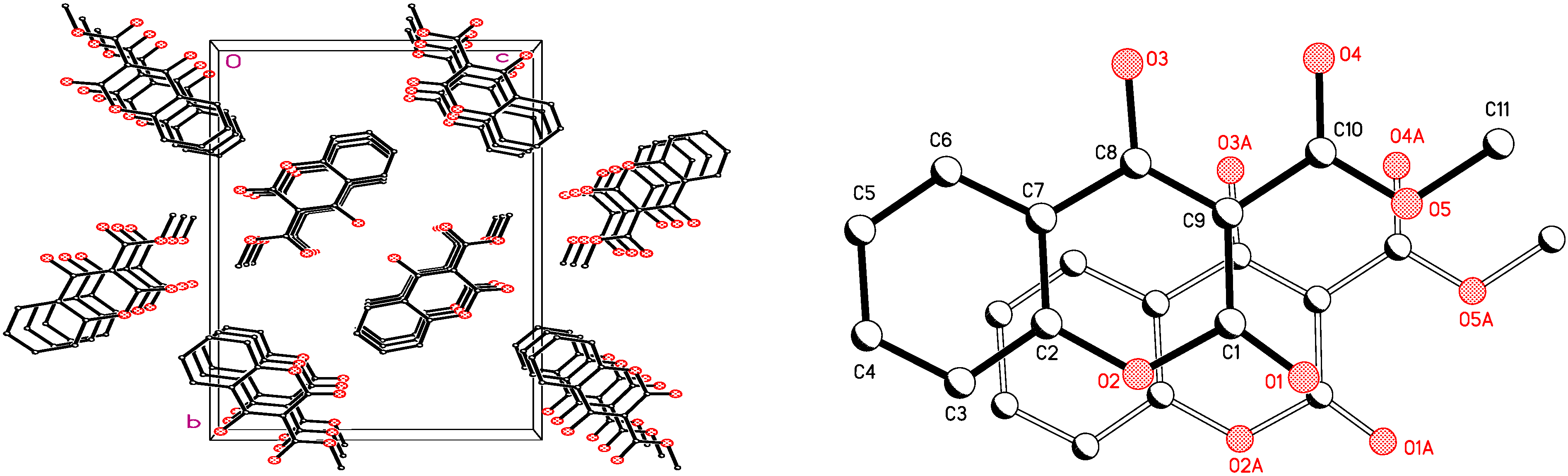
| C(1)-O(1) | 1.199(4) | O(2)-C(2)-C(3) | 116.7(4) |
| C(1)-O(2) | 1.378(5) | O(2)-C(2)-C(7) | 121.9(4) |
| C(1)-C(9) | 1.450(5) | C(3)-C(2)-C(7) | 121.4(4) |
| O(2)-C(2) | 1.371(5) | C(2)-C(3)-C(4) | 118.9(4) |
| C(2)-C(3) | 1.374(6) | C(3)-C(4)-C(5) | 120.6(4) |
| C(2)-C(7) | 1.384(6) | C(6)-C(5)-C(4) | 120.5(4) |
| C(3)-C(4) | 1.376(6) | C(5)-C(6)-C(7) | 119.3(4) |
| C(4)-C(5) | 1.387(6) | C(2)-C(7)-C(6) | 119.3(4) |
| C(5)-C(6) | 1.372(6) | C(2)-C(7)-C(8) | 117.3(3) |
| C(6)-C(7) | 1.398(6) | C(6)-C(7)-C(8) | 123.4(4) |
| C(7)-C(8) | 1.435(6) | O(3)-C(8)-C(9) | 123.3(4) |
| C(8)-O(3) | 1.310(5) | O(3)-C(8)-C(7) | 115.6(3) |
| C(8)-C(9) | 1.375(5) | C(9)-C(8)-C(7) | 121.1(3) |
| C(9)-C(10) | 1.457(6) | C(8)-C(9)-C(1) | 120.0(3) |
| C(10)-O(4) | 1.232(5) | C(8)-C(9)-C(10) | 118.3(3) |
| C(10)-O(5) | 1.317(5) | C(1)-C(9)-C(10) | 121.7(3) |
| O(5)-C(11) | 1.441(5) | O(4)-C(10)-O(5) | 121.8(4) |
| O(1)-C(1)-O(2) | 115.0(3) | O(4)-C(10)-C(9) | 121.9(4) |
| O(1)-C(1)-C(9) | 127.8(4) | O(5)-C(10)-C(9) | 116.3(3) |
| O(2)-C(1)-C(9) | 117.2(3) | C(10)-O(5)-C(11) | 116.4(3) |
| C(2)-O(2)-C(1) | 122.4(3) |
| D-H...A | d(D-H) | d(H...A) | D(D...A) | <(DHA) |
| O(3)-H(3A)...O(4) | 0.84 | 1.77 | 2.512(4) | 146.6 |
2.2. Quantum Chemical Calculations
2.3. Computational Studies
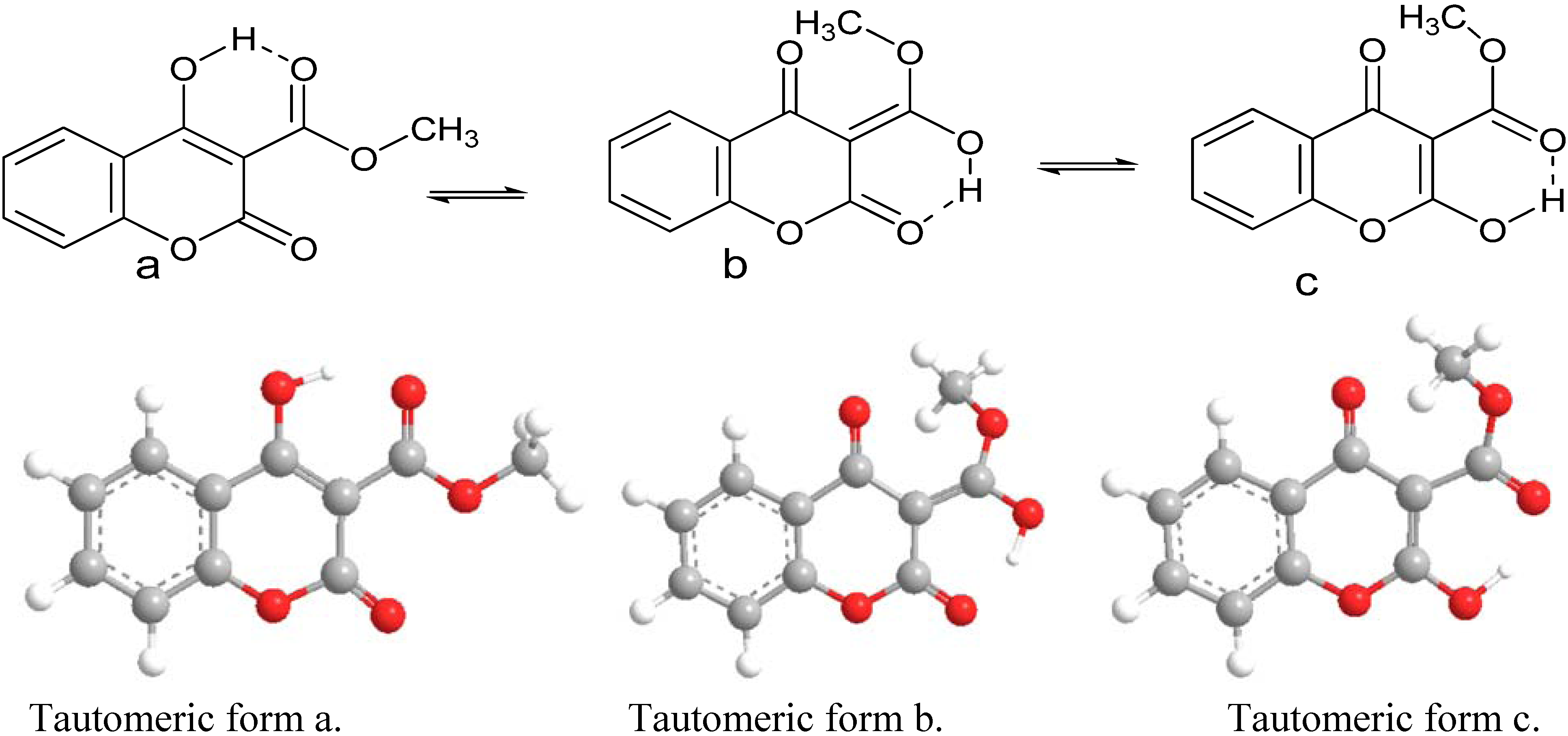
| Total Energy (kcal/mol) | ΔΕ | |
| Tautomer a | 502236.25 | 0 |
| Tautomer b | 502222.04 | 14.21 |
| Tautomer c | 502221.33 | 14.92 |
| Bonds (Å) | X-ray | DFT |
| C(1)-O(1) | 1.199 | 1.198 |
| C(8)-O(3) | 1.310 | 1.319 |
| C(10)-O(4) | 1.232 | 1.238 |
| O(2)-C(2) | 1.371 | 1.356 |
| C(10)-O(5) | 1.317 | 1.323 |
| C(1)-C(9) | 1.450 | 1.463 |
| C(9)-C(10) | 1.457 | 1.469 |
| C(8)-C(9) | 1.375 | 1.394 |
| Angles (°) | X-ray | DFT |
| O(1)-C(1)-O(2) | 115 | 115.9 |
| C(9)-C(10)-O(5) | 116.3 | 116.3 |
| C(10)-O(5)-C(11) | 116.3 | 116.6 |
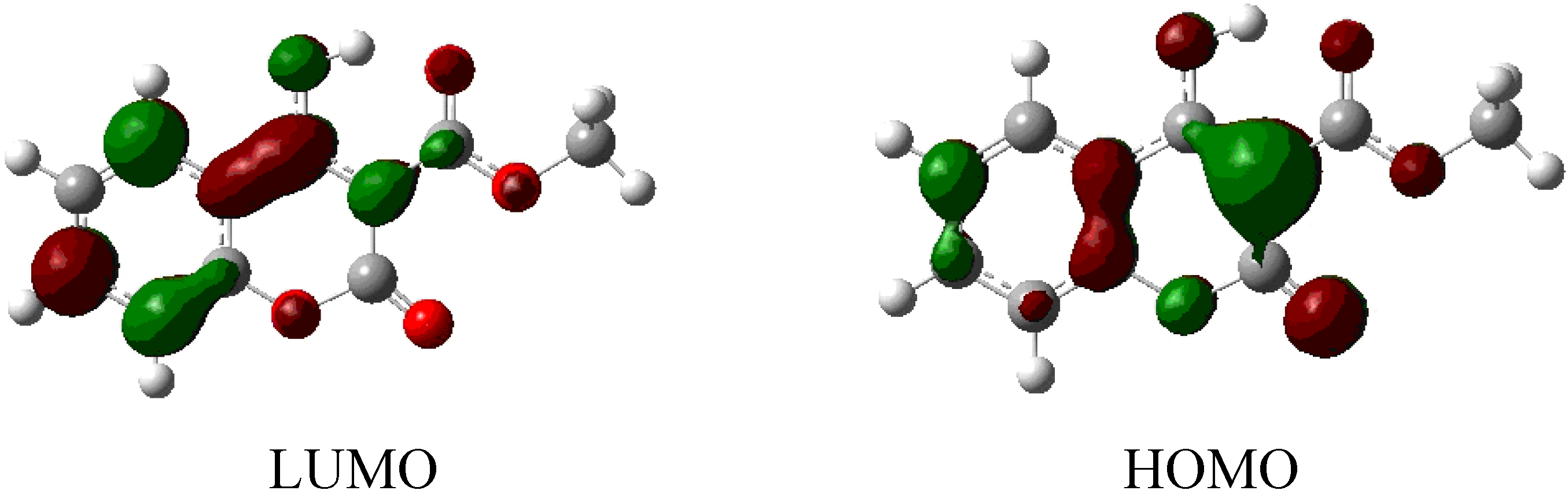
| Orbital | Energy (eV) |
|---|---|
| LUMO+3 | −0.01268 |
| LUMO+2 | −0.01715 |
| LUMO+1 | −0.03766 |
| LUMO | −0.09207 |
| HOMO | −0.25767 |
| HOMO-1 | −0.27303 |
| HOMO-2 | −0.28980 |
| HOMO-3 | −0.30505 |
| Total Energy (kcal/mol) | ΔΕ | |
|---|---|---|
| Tautomer a | 502251.36 | 0 |
| Tautomer b | 502236.36 | 15.00 |
| Tautomer c | 502236.08 | 15.28 |
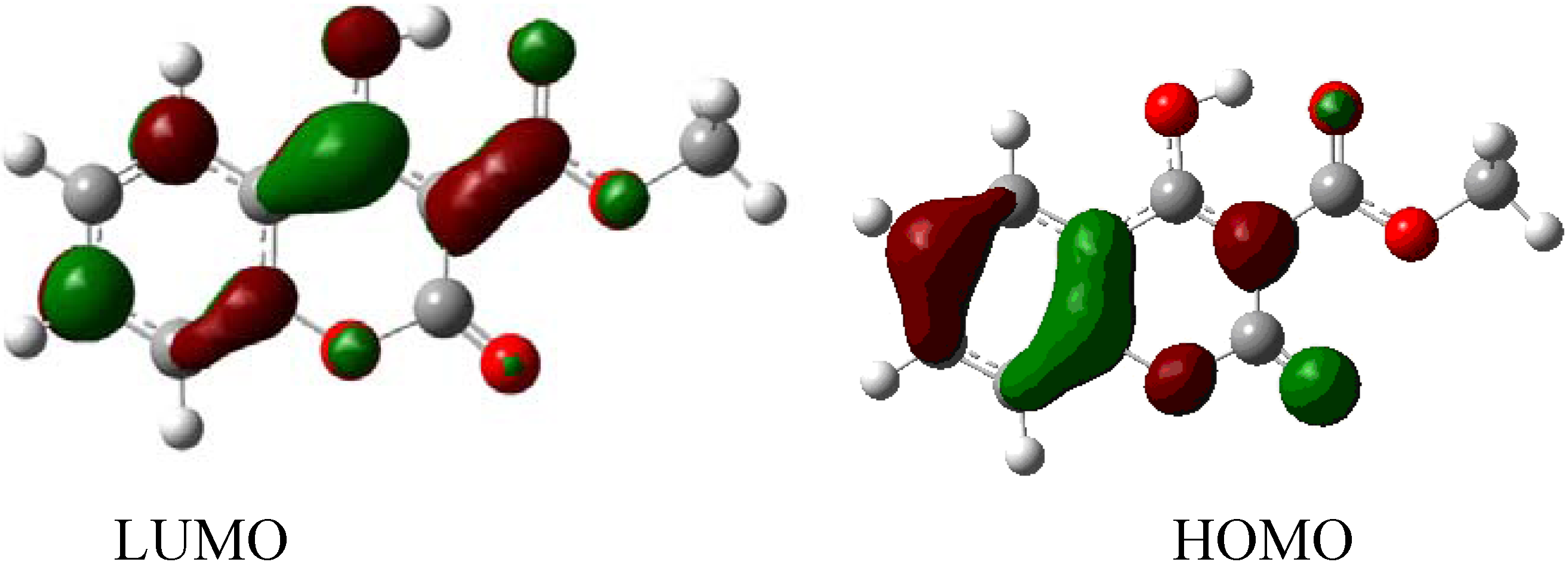
3. Experimental
3.1. General
3.1.1. General Procedure for the Synthesis of the N-hydroxysuccinimide Ester of O-acetylsalicylic Acid (2)
3.1.2. General Procedure for the Synthesis of 3-Substituted-4-Hydroxycoumarins
3.2. Crystal Structure Determination of 4a
4. Conclusions
Supporting Information
| Center Number | Atomic Number | Atomic Type | Coordinates (Angstroms) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 1 | 6 | 0 | −3.863365 | −1.026907 | 0.000004 |
| 2 | 6 | 0 | −4.135989 | 0.349539 | 0.000006 |
| 3 | 6 | 0 | −3.107716 | 1.279694 | 0.000004 |
| 4 | 6 | 0 | −1.785738 | 0.829994 | 0.000001 |
| 5 | 6 | 0 | −1.495749 | −0.540269 | −0.000001 |
| 6 | 6 | 0 | −2.552628 | −1.468517 | 0.000001 |
| 7 | 8 | 0 | −0.806136 | 1.767112 | −0.000002 |
| 8 | 6 | 0 | 0.568307 | 1.458477 | −0.000005 |
| 9 | 6 | 0 | 0.902676 | 0.034388 | −0.000002 |
| 10 | 6 | 0 | −0.105367 | −0.929393 | −0.000003 |
| 11 | 8 | 0 | 0.138342 | −2.225277 | −0.000005 |
| 12 | 6 | 0 | 2.292934 | −0.439924 | −0.000002 |
| 13 | 8 | 0 | 3.231744 | 0.492372 | 0.000011 |
| 14 | 6 | 0 | 4.599130 | 0.032938 | 0.000012 |
| 15 | 8 | 0 | 1.315559 | 2.394467 | −0.000013 |
| 16 | 8 | 0 | 2.573797 | −1.645763 | −0.000009 |
| 17 | 1 | 0 | −4.677724 | −1.740938 | 0.000006 |
| 18 | 1 | 0 | −5.163665 | 0.694595 | 0.000009 |
| 19 | 1 | 0 | −3.298328 | 2.345344 | 0.000005 |
| 20 | 1 | 0 | −2.316874 | −2.524743 | 0.000000 |
| 21 | 1 | 0 | 1.136344 | −2.329785 | −0.000006 |
| 22 | 1 | 0 | 5.197386 | 0.940375 | 0.000023 |
| 23 | 1 | 0 | 4.798717 | −0.564118 | −0.890153 |
| 24 | 1 | 0 | 4.798710 | −0.564134 | 0.890168 |
| Center Number | Atomic Number | Atomic Type | Coordinates (Angstroms) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 1 | 6 | 0 | −3.618148 | −1.245874 | −0.007977 |
| 2 | 6 | 0 | −3.998088 | 0.078140 | 0.245068 |
| 3 | 6 | 0 | −3.047740 | 1.089629 | 0.295254 |
| 4 | 6 | 0 | −1.709017 | 0.761144 | 0.096863 |
| 5 | 6 | 0 | −1.304270 | −0.549857 | −0.147866 |
| 6 | 6 | 0 | −2.281357 | −1.553277 | −0.207628 |
| 7 | 8 | 0 | −0.810933 | 1.805230 | 0.121572 |
| 8 | 6 | 0 | 0.524803 | 1.604765 | −0.018278 |
| 9 | 6 | 0 | 1.041859 | 0.251884 | −0.085742 |
| 10 | 6 | 0 | 0.131388 | −0.866082 | −0.340040 |
| 11 | 8 | 0 | 0.488894 | −1.980777 | −0.710457 |
| 12 | 6 | 0 | 2.457879 | 0.140135 | −0.089479 |
| 13 | 8 | 0 | 3.172725 | −0.945992 | 0.051319 |
| 14 | 8 | 0 | 3.217744 | 1.187155 | −0.227783 |
| 15 | 6 | 0 | 2.764139 | −2.092598 | 0.836929 |
| 16 | 8 | 0 | 1.214329 | 2.624762 | −0.071247 |
| 17 | 1 | 0 | −4.368400 | −2.026310 | −0.049685 |
| 18 | 1 | 0 | −5.042901 | 0.322058 | 0.399237 |
| 19 | 1 | 0 | −3.318669 | 2.122060 | 0.476953 |
| 20 | 1 | 0 | −1.954487 | −2.565527 | −0.411849 |
| 21 | 1 | 0 | 2.597225 | 1.992583 | −0.271100 |
| 22 | 1 | 0 | 3.683895 | −2.441204 | 1.303092 |
| 23 | 1 | 0 | 2.043747 | −1.791336 | 1.597193 |
| 24 | 1 | 0 | 2.328808 | −2.843394 | 0.186302 |
| Center Number | Atomic Number | Atomic Type | Coordinates (Angstroms) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 1 | 6 | 0 | 3.625545 | −1.219490 | −0.028375 |
| 2 | 6 | 0 | 3.991918 | 0.105914 | 0.240048 |
| 3 | 6 | 0 | 3.030236 | 1.105326 | 0.304629 |
| 4 | 6 | 0 | 1.698956 | 0.755070 | 0.102571 |
| 5 | 6 | 0 | 1.303332 | −0.553836 | −0.159337 |
| 6 | 6 | 0 | 2.293164 | −1.543919 | −0.230248 |
| 7 | 8 | 0 | 0.776087 | 1.783523 | 0.141362 |
| 8 | 6 | 0 | −0.522396 | 1.518830 | 0.014305 |
| 9 | 6 | 0 | −1.048824 | 0.229110 | −0.096703 |
| 10 | 6 | 0 | −0.132598 | −0.880766 | −0.357093 |
| 11 | 8 | 0 | −0.480906 | −1.995785 | −0.729157 |
| 12 | 6 | 0 | −2.523855 | 0.155251 | −0.147462 |
| 13 | 8 | 0 | −3.193606 | 1.163428 | −0.399711 |
| 14 | 8 | 0 | −1.227759 | 2.608730 | 0.006259 |
| 15 | 8 | 0 | −3.196434 | −0.963313 | 0.091663 |
| 16 | 6 | 0 | −2.723093 | −2.031505 | 0.940363 |
| 17 | 1 | 0 | 4.385801 | −1.989630 | −0.080094 |
| 18 | 1 | 0 | 5.034104 | 0.359492 | 0.394986 |
| 19 | 1 | 0 | 3.287543 | 2.138811 | 0.499467 |
| 20 | 1 | 0 | 1.978969 | −2.557818 | −0.445769 |
| 21 | 1 | 0 | −2.176000 | 2.299376 | −0.206636 |
| 22 | 1 | 0 | −3.600399 | −2.352457 | 1.500562 |
| 23 | 1 | 0 | −1.956518 | −1.673761 | 1.628696 |
| 24 | 1 | 0 | −2.326874 | −2.836595 | 0.329283 |
Acknowledgements
References and Notes
- Murray, R.D.H.; Mendez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry and Biochemistry; Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Naser-Hijazi, B.; Stolze, B.; Zanker, K.S. 2nd Proceedings of the International Society of Coumarin Investigators; Springer: Berlin, Germany, 1994. [Google Scholar]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Hepworth, J.D. Comprehensive Heterocyclic Chemistry; Katritzky, A., Rees, C.W., Boulton, A.J., McKillop, A., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 3, pp. 799–810, Chapter 2.24. [Google Scholar]
- Jung, J.C.; Jung, Y.J.; Park, O.S. A Convenient one-pot synthesis of 4-hydroxycoumarin, 4-hydroxythiocoumarin and 4-hydroxyquinolin-2(1H)-one. Synth. Commun. 2001, 31, 1195–1200. [Google Scholar] [CrossRef]
- Gnerre, C.; Catto, M.; Leonetti, F.; Weber, P.; Carrupt, P.A.; Altomare, C.; Carotti, A.; Testa, B. Inhibition of monoamine oxidases by functionalized coumarin derivatives: biological activities, QSARs, and 3D-QSARs. J. Med. Chem. 2000, 43, 4747–4758. [Google Scholar] [CrossRef]
- Zahradnik, M. The Production and Application of Fluoroscent Brightening Agents; Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Hesse, S.; Kirsch, G. A rapid access to coumarin derivatives using Vilsmeier-Haack and Suzuki cross-coupling reactions. Tetrahedron Lett. 2002, 43, 1213–1215. [Google Scholar] [CrossRef]
- Singer, L.A.; Kong, N.P. Vinyl Radicals. Stereoselectivity in hydrogen atom transfer to equilibated isomeric vinyl radicals. J. Am. Chem. Soc. 1966, 88, 5213–5219. [Google Scholar] [CrossRef]
- Carboni, S.; Malaguzzi, V.; Marzili, A. Ferulenol a new coumarin derivative from ferula communis. Tetrahedron Lett. 1964, 5, 2783–2785. [Google Scholar] [CrossRef]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-Zinc Mediated Inhibition of Carbonic Anhydrases: Coumarins Are a New Class of Suicide Inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar]
- Nolan, K.A.; Doncaster, J.R.; Dunstan, M.S.; Scott, K.A.; Frenkel, A.D.; Siegel, D.; Ross, D.; Barnes, J.; Levy, C.; Leys, D.; Whitehead, R.C.; Stratford, I.J.; Bryce, R.A. Synthesis and biological evaluation of coumarin-based inhibitors of NAD(P)H: quinone oxidoreductase-1 (NQO1). J. Med. Chem. 2009, 52, 7142–7156. [Google Scholar]
- Nolan, K.A.; Zhao, H.; Faulder, P.F.; Frenkel, A.D.; Timson, D.J.; Siegel, D.; Ross, D.; Burke, T.R., Jr.; Stratford, I.J.; Bryce, R.A. Coumarin-based inhibitors of human NAD(P)H:quinone oxidoreductase-1. Identification, structure-activity, off-target effects and in vitro human pancreatic cancer toxicity. J. Med. Chem. 2007, 50, 6316–6325. [Google Scholar] [CrossRef]
- Cai, S.X.; Zhang, H.; Kemmitzer, W.E.; Jiang, S.; Drewe, J.A.; Storer, R. PCT Int. Appl. WO 2002092076 A1 20021121, 2002.
- Wagner, B.D. The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef]
- Scypinski, S.; Drake, J.M. Photophysics of Coumarin Inclusion Complexes with Cyclodextrin. Evidence for Normal and Inverted Complex Formation. J. Phys. Chem. 1985, 89, 2432–2435. [Google Scholar] [CrossRef]
- Barooah, N.; Pemberton, B.C.; Johnson, A.C.; Sivaguru, J. Photodimerization and Complexation Dynamics of Coumarins in the Presence of Cucurbit[8]urils. Photochem. Photobiol. Sci. 2008, 7, 1473–1479. [Google Scholar] [CrossRef]
- Hirsh, J.; Dalen, J.; Anderson, D.R.; Poller, L.; Bussey, H.; Ansel, J.; Deykin, D. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001, 119 (suppl 1), 8S–21S. [Google Scholar] [CrossRef]
- Cesar, J.M.; Garcia-Avello, A.; Navarro, J.L.; Herraez, M.V. Aging and oral anticoagulant therapy using acenocoumarol. Blood Coagul. Fibrinolysis 2004, 15, 673–676. [Google Scholar]
- Manolov, I.; Maichle-Moessmer, C.; Danchev, N. Synthesis, structure, toxicological and pharmacological investigations of 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2006, 41, 882–890. [Google Scholar] [CrossRef]
- Gebauer, M. Synthesis and structure-activity relationships of novel warfarin derivatives. Bioorg. Med. Chem. 2007, 15, 2414–2420. [Google Scholar] [CrossRef]
- Trivedi, J.C.; Bariwal, J.B.; Upadhyay, K.D.; Naliapara, Y.T.; Joshi, S.K.; Pannecouque, C.C.; De Clercq, E.; Shah, A.K. Improved and rapid synthesis of new coumarinyl chalcone derivatives and their antiviral activity. Tetrahedron Lett. 2007, 48, 8472–8474. [Google Scholar]
- Stanchev, S.; Momekov, G.; Jensen, F.; Manolov, I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2008, 43, 694–706. [Google Scholar] [CrossRef]
- Radanyi, C.; Le Bras, G.; Massaoudi, S.; Bouclier, C.; Peyrat, J.F.; Brion, J.D.; Marsaud, V.; Renoir, J.M.; Alami, M. Synthesis and biological activity of simplified denoviose-coumarins related to novobiocin as potent inhibitors of heart-shock protein 90 (hsp90). Bioorg. Med. Chem. Lett. 2008, 18, 2495–2498. [Google Scholar]
- Stanchev, S.; Hdjimitova, V.; Traykov, T.; Boyanov, T.; Manolov, I. Investigation of the antioxidant properties of some new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2009, 44, 3077–3082. [Google Scholar] [CrossRef]
- Park, O.S.; Jang, B.S. Synthesis of 4-hydroxycoumarin derivatives-1: An efficient synthesis of flocoumafen. Arch. Pharm. Res. 1995, 18, 277–281. [Google Scholar] [CrossRef]
- Lamnaouer, D.; Omari, M.; Mounir, A.; El Alouani, M. Activité anti-coagulante de. F. communis L. chez le monton. Maghreb Veterinaire 1990, 5, 5–10. [Google Scholar]
- Valle, M.G.; Appendino, G.; Nano, G.M.; Picci, V. Prenylated coumarins and sesquiterpenoids from Ferula communis. Phytochemistry 1987, 26, 253–256. [Google Scholar]
- Lamnaouer, D.; Martin, M.T.; Bodo, B.; Molho, D. Lipid componenets of the seagrasses Posidonia australis and Heterozostera tasmanica as indicators of carbon source. Phytochemistry 1987, 26, 1613–1621. [Google Scholar]
- Appendino, G.; Tagliapietra, S.; Nano, G.M.; Picci, V. Ferprenin, a prenylated coumarin from Ferula communis. Phytochemistry 1988, 27, 944–946. [Google Scholar]
- Appendino, G.; Tagliapietra, S.; Cariboldi, P.; Nano, G.M.; Picci, V. ω-Oxygenated prenylated coumarins from Ferula communis. Phytochemistry 1988, 27, 3619–3624. [Google Scholar]
- Miski, M.; Jakupovic, J. Cyclic farnesyl-coumarin and farnesyl-chromone derivatives from Ferula communis subsp. Communis. Phytochemsitry 1990, 29, 1995–1998. [Google Scholar]
- Lamnaouer, D.; Fraigui, O.; Martin, M.T.; Bodo, B. Structure of ferulenol derivatives from Ferula communis var genuine. Phytochemistry 1991, 30, 2383–2386. [Google Scholar]
- Saidkhodzhaev, A.I.; Kushmuradov, A.Y.; Malikov, V.M. Fepaldine-Terpenoid Coumarin from Ferrula-Pallida. Khim. Prir. Soedin. 1980, 6, 716–718. [Google Scholar]
- Su, B.-N.; Takaishi, Y.; Honda, G.; Itoh, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O. Sesquiterpene coumarins and related derivatives from Ferulla pallida. J. Nat. Prod. 2000, 63, 436–440. [Google Scholar] [CrossRef]
- Lee, B.H.; Clothier, M.F.; Dutton, F.E.; Conder, G.A.; Johnson, S.S. Anthelmintic β-hydroxyketoamides (BKAs). Bioorg. Med. Chem. Lett. 1998, 8, 3317–3320. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, S.P.; Gao, H.; Babu, M.S.; Debnath, A.K.; Hansch, C. Comparatative Quantitative Structure-Activity Relationship studies on Anti-HIV drugs. Chem. Rev. 1999, 99, 3525–3602. [Google Scholar] [CrossRef]
- von Pechmann, H. Neue Bildungsweise der Cumarine. Synthese des Daphnetins. Chem. Ber. 1884, 17, 929. [Google Scholar] [CrossRef]
- Yavari, I.; Hekmat-Shoar, R.; Zonouzi, A. A new and efficient route to 4-carboxymethylcoumarins mediated by vinyltriphenylphosphonium salt. Tetrahedron Lett. 1998, 39, 2391–2392. [Google Scholar] [CrossRef]
- Rodriguez, I.; Iborra, S.; Rey, F.; Corma, A. Heterogenized Bronsted base catalysts for fine chemicals production: grafted quaternary organic ammonium hydroxides as catalyst for the production of chromenes and coumarins. Appl. Catal. Gen. 2000, 194-195, 241–252. [Google Scholar] [CrossRef]
- Geoghegan, M.; O’Sullivan, W.I.; Philbin, E.M. Flavonoid epoxides-II: A new synthesis of 4-hydroxy-3-phenylcoumarins. Tetrahedron 1966, 22, 3209–3211. [Google Scholar] [CrossRef]
- Jain, A.C.; Rohatgi, V.K.; Seshadri, T.R. A novel synthesis of 3-phenyl-4-hydroxycoumarins. Tetrahedron Lett. 1966, 7, 2701–2705. [Google Scholar] [CrossRef]
- Jain, A.C.; Rohatgi, V.K.; Seshadri, T.R. A novel synthesis of 3-phenyl-4-hydroxycoumarins. Tetrahedron 1967, 23, 2499–2504. [Google Scholar] [CrossRef]
- Kalinin, A.V.; da Silva, A.J.M.; Lopes, C.C.; Lopes, R.S.C.; Snieckus, V. Directed ortho metalation-cross coupling links. Carbamoyl rendition of the Baker-Venkataraman rearrangement. Regiospecific route to substituted 4-hydroxycoumarins. Tetrahedron Lett. 1998, 39, 4995–4998. [Google Scholar]
- Liu, Y.; Kurth, M.J. Ipso substitution as a route to Benzo[c]quinolizines and 4-hydroxycoumarins. J. Org. Chem. 2002, 67, 2082–2086. [Google Scholar] [CrossRef]
- Jung, J.-C.; Kim, J.-C.; Park, O.-S. Simple and cost-effective syntheses of 4-hydroxycoumarins. Synth. Commun. 1999, 29, 3587–3595. [Google Scholar] [CrossRef]
- Athanasellis, G.; Melagraki, G.; Chatzidakis, H.; Afantitis, A.; Detsi, A.; Igglessi-Markopoulou, O.; Markopoulos, J. Novel short-step synthesis of functionalized γ-hydroxybutenoates and their cyclization 4-hydroxy coumarins via the N-hydroxybenzotriazole methodology. Synthesis 2004, 11, 1775–1782. [Google Scholar]
- Mitsos, C.; Zografos, A.L.; Igglessi-Markopoulou, O. Synthesis of 3-substituted-4-hydroxy-quinolin-2-ones via C-acylation reactions of active methylene compounds with functionalized 3,1-benzoxazin-4-ones. Heterocycles 1999, 51, 1543. [Google Scholar] [CrossRef]
- Zikou, L.; Athanasellis, G.; Detsi, A.; Zografos, A.; Mitsos, C.; Igglessi-Markopoulou, O. A novel short-step synthesis of functionalized 4-hydroxy-2-quinolinones using the N-hydroxybenzotriazole methodology. Bull Chem. Soc. Jpn. 2004, 77, 1505–1508. [Google Scholar] [CrossRef]
- Athanasellis, G.; Melagraki, G.; Afantitis, A.; Makridima, K.; Igglessi-Markopoulou, O. A simple synthesis of functionalized 2-amino-3-cyano-4-chromones by applying the N-Hydroxybenzotriazole methodology. ARKIVOC 2006, X, 28–34. [Google Scholar]
- Kikionis, S.; McKee, V.; Markopoulos, J.; Igglessi-Markopoulou, O. A prominent C-acylation-cyclisation synthetic sequence and X-ray structure elucidation of benzothiopyranone derivatives. Tetrahedron 2008, 64, 5454–5458. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Porter, W.R.; Trager, W.F. 4-Hydroxycoumarin/2-Hydroxychromone Tautomerism: Infrared Spectra of 3-Substituted-2-13C-4-hydroxycoumarins. J. Heterocyclic Chem. 1982, 19, 475–480. [Google Scholar] [CrossRef]
- Porter, W.R. Warfarin: history, tautomerism and activity. J. Comp. Aided Mol. Des. 2010, 24, 553–573. [Google Scholar] [CrossRef]
- Cave, R.J.; Burke, K.; Castner, E.W., Jr. Theoretical Investigations of the Ground and Excited States of Coumarin 151 and Coumarin 120. J. Phys. Chem. A 2002, 106, 9294–9305. [Google Scholar]
- Georgieva, I.; Trendafilova, N.; Aquino, A.; Lischka, H. Excited State Properties of 7-Hydroxy-4-methylcoumarin in the Gas Phase and in Solution A Theoretical Study. J. Phys. Chem. A 2005, 109, 11860–11869. [Google Scholar] [CrossRef]
- Improta, R.; Barone, V.; Scalmani, G.; Frisch, M.J. A state-specific polarizable continuum model time dependent density functional theory method for excited state calculations in solution. J. Chem. Phys. 2006, 125, 054103–054112. [Google Scholar] [CrossRef]
- Jacquemin, D.; Perpete, E.A.; Scalmani, G.; Frisch, M.J.; Assfeld, X.; Ciofini, I.; Adamo, C. Time-dependent density functional theory investigation of the absorption, fluorescence, and phosphorescence spectra of solvated coumarins. J. Chem. Phys. 2006, 125, 164324–164335. [Google Scholar] [CrossRef]
- Kurashige, Y.; Nakajima, T.; Kurashige, S.; Hirao, K.; Nishikitani, Y. Theoretical Investigation of the Excited States of Coumarin Dyes for Dye-Sensitized Solar Cells. J. Phys. Chem. A 2007, 111, 5544–5548. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Malecka, M.; Modranka, R.; Budzisz, E. Synthesis, structural and conformational study of chromane derivatives. J. Mol. Struct. 2009, 937, 139–145. [Google Scholar]
- Lameira, J.; Alves, C.N.; Santos, L.S.; Santos, A.S.; de Almeida Santos, R.H.; Souza, J., Jr.; Silva, C.C.; da Silva, A.B.F. A combined X-ray and theoretical study of flavonoid compounds with anti-inflammatory activity. J. Mol. Struct. (TheoChem) 2008, 862, 16–20. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhao, P.-S.; Li, R.-Q.; Zhou, S.-M. Synthesis, Crystal Structure and Quantum Chemical Study on 3-Phenylamino-4-Phenyl-1,2,4-Triazole-5-Thione. Molecules 2009, 14, 608–620. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09W, A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Preat, J.; Jacquemin, D.; Wathelet, V.; André, J.-M.; Perpète, E.A. TD-DFT Investigation of the UV Spectra of Pyranone Derivatives. J. Phys. Chem. A 2006, 110, 8144–8150. [Google Scholar]
- Zou, W.; Gao, Y.; Sugiyama, T.; Matsuura, T.; Meng, J. Succinimido 2-acetoxybenzoate. Acta Crystallogr. E 2003, o80–o83. [Google Scholar]
- Sample Availability: Samples of the compounds 2, 3a-c, 4a-d are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stefanou, V.; Matiadis, D.; Melagraki, G.; Afantitis, A.; Athanasellis, G.; Igglessi-Markopoulou, O.; McKee, V.; Markopoulos, J. Functionalized 4-Hydroxy Coumarins: Novel Synthesis, Crystal Structure and DFT Calculations. Molecules 2011, 16, 384-402. https://doi.org/10.3390/molecules16010384
Stefanou V, Matiadis D, Melagraki G, Afantitis A, Athanasellis G, Igglessi-Markopoulou O, McKee V, Markopoulos J. Functionalized 4-Hydroxy Coumarins: Novel Synthesis, Crystal Structure and DFT Calculations. Molecules. 2011; 16(1):384-402. https://doi.org/10.3390/molecules16010384
Chicago/Turabian StyleStefanou, Valentina, Dimitris Matiadis, Georgia Melagraki, Antreas Afantitis, Giorgos Athanasellis, Olga Igglessi-Markopoulou, Vickie McKee, and John Markopoulos. 2011. "Functionalized 4-Hydroxy Coumarins: Novel Synthesis, Crystal Structure and DFT Calculations" Molecules 16, no. 1: 384-402. https://doi.org/10.3390/molecules16010384




