Schistosomicidal Activity of the Essential Oil of Ageratum conyzoides L. (Asteraceae) against Adult Schistosoma mansoni Worms
Abstract
:1. Introduction
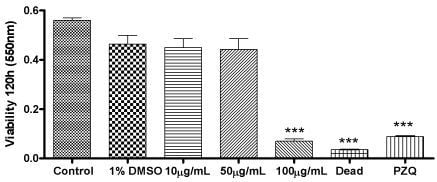
2. Results and Discussion
3. Experimental
3.1. Plant material
3.2. Chemicals
3.3. GC-MS analysis
3.3. Parasite culture and maintenance
3.4. In vitro studies with Schistosoma mansoni
3.5. Viability assay
3.6. Statistical analysis
4. Conclusions
Acknowledgements
References
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Ottesen, E.; Sachs, S.E.; Sachs, J.D. Incorporating a Rapid-Impact Package for Neglected Tropical Diseases with Programs for HIV/AIDS, Tuberculosis, and Malaria. PLOS Med. 2006, 3, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Magalhães, L.G.; Kapadia, G.J.; Tonuci, L.R.S.; Caixeta, S.C.; Parreira, N.A.; Rodrigues, V.; Da Silva Filho, A.A. In vitro schistosomicidal effects of some phloroglucinol derivatives from Dryopteris species against Schistosoma mansoni adult worms. Parasitol. Res. 2010, 106, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.J.; McManus, D.P.; Li, Y.; Williams, G.M.; Bergquist, R.; Gross, A.G. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect. Dis. 2010, 10, 733–736. [Google Scholar] [CrossRef]
- Jiwajinda, S.; Santisopasri, V.; Murakami, A.; Kawanaka, M.; Kawanaka, H.; Gasquet, M.; Eilas, R.; Balansard, G.; Ohigashi, H. In vitro anti-tumor promoting and anti-parasitic activities of the quassinoids from Eurycoma longifolia, a medicinal plant in Southeast Asia. J. Ethnopharmacol. 2002, 82, 55–58. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.; El-Nahas, H.A.; Abo-Sedera, S.A. Antischistosomal and antimicrobial activities of some Egyptian plant species. Pharm. Biol. 2008, 46, 626–633. [Google Scholar] [CrossRef]
- Ismail, M.; Botros, S.; Metwally, A.; William, S.; Farghally, A.; Tao, L.F.; Day, T.A.; Bennett, J.L. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999, 60, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Jiwajinda, S.; Santisopasri, V.; Murakami, A.; Kawanaka, M.; Kawanaka, H.; Gasquet, M.; Eilas, R.; Balansard, G.; Ohigashi, H. In vitro anti-tumor promoting and anti-parasitic activities of the quassinoids from Eurycoma longifolia, a medicinal plant in Southeast Asia. J. Ethnopharmacol. 2002, 82, 55–58. [Google Scholar] [CrossRef]
- Parreira, N.A.; Magalhães, L.G.; Moraisa, D.R.; Caixeta, S.C.; de Sousa, J.P.B.; Bastos, J.K.; Cunha, W.R.; Silva, M.L.A.; Nanayakkara, N.P.D.; Rodrigues, V.; da Silva Filho, A.A. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia. Chem. Biodivers. 2010, 7, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Crotti, A.E.M.; Dos Santos, F.; Magalhães, L.G.; Wakabayashi, K.A.L.; Aguiar, G.P.; Carvalho, C.E.; Melo, N.I.; Veneziani, R.; Albuquerque, S.; Silva-Filho, A.A. Screening of selected essential oils for their in vitro antileishmanial activity against Leishmania amazonensis. Planta Med. 2010, 76, 1307–1307. [Google Scholar] [CrossRef]
- Ekundayo, O.; Laakso, I.; Hiltunen, R. Essential oil of Ageratum conyzoides. Planta Med. 1988, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Saluja, A.K. Ageratum conyzoides L.: A review on its phytochemical and pharmacological profile. Int. J. Green Pharm. 2008, 2, 59–68. [Google Scholar] [CrossRef]
- Lima, M.A.S.; Barros, M.C.P.; Pinheiro, S.M.; do Nascimento, R.F.; Matos, F.J.D.; Silveira, E.R. Volatile compositions of two Asteraceae from the North-east of Brazil: Ageratum conyzoides and Acritopappus confertus (Eupatorieae). Flavour Frag. J. 2005, 20, 559–561. [Google Scholar] [CrossRef]
- Boudaa, H.; Tapondjoua, L.A.; Fontemb, D.A.; Gumedzoec, M.Y.D. Effect of essential oils from leaves of Ageratum conyzoides, Lantana camara and Chromolaena odorata on the mortality of Sitophilus zeamais (Coleoptera, Curculionidae). J. Stored. Prod. Res. 2001, 37, 103–109. [Google Scholar] [CrossRef]
- Lima, R.K.; Cardoso, M.D.; Moraes, J.C.; Andrade, M.A.; Melo, B.A.; Rodrigues, V.G. Chemical characterization and insecticidal activity of the essential oil leaves of Ageratum conyzoides L. on fall armyworm Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae). Biosci. J. 2010, 26, 1–5. [Google Scholar]
- Dixit, S.N.; Chandra, H.; Tiwari, R.; Dixit, V. Development of a botanical fungicide against blue mould of Mandarins. J. Stored Prod. Res. 1995, 31, 165–172. [Google Scholar] [CrossRef]
- Nogueira, J.H.C.; Goncalez, E.; Galleti, S.R.; Facanali, R.; Marques, M.O.M.; Felicio, J.D. Ageratum conyzoides essential oil as aflatoxin suppressor of Aspergillus flavus. Int. J. Food Microbiol. 2010, 137, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.C.A.; Silva, E.L.F.; Fraga, M.C.A.; Wanderley, A.G.; Afiatpour, P.; Maia, M.B.S. Antiinflammatory and chronic toxicity study of the leaves of Ageratum conyzoides L. in rats. Phytomedicine 2005, 12, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Momesso, L.S.; de Moura, R.M.X.; Constantino, D.H.J. Antitumoral activity of Ageratum conyzoides L. (Asteraceae). Rev. Bras. Farmacogn. 2009, 19, 660–663. [Google Scholar] [CrossRef]
- De Oliveira-Penido, M.L.; Zech-Coelho, P.M.; de Mello, R.T.; Piló-Veloso, D.; de Oliveira, M.C.; Kusel, J.R.; Nelson, D.L. Antischistosoma activity of aminoalkanethiols, alkylaminoalkanethiosulfuric acids and the corresponding disulfides. Acta Trop. 2008, 108, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Boissier, J.; Coslédan, F.; Robert, A.; Meunier, B. In vitro activities of trioxaquines against Schistosoma mansoni. Antimicrob. Agents Ch. 2009, 53, 4903–4906. [Google Scholar] [CrossRef] [PubMed]
- Botros, S.S.; William, S.; Beadle, J.R.; Valiaeva, N.; Hostetler, K.Y. Antischistosomal activity of hexadecyloxypropyl cyclic 9-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine and other alkoxyalkyl esters of acyclic nucleoside phosphonates assessed by schistosome worm killing in vitro. Antimicrob. Agents Ch. 2009, 53, 5284–5287. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.G.; Machado, C.B.; Morais, E.R.; Moreira, E.B.C.; Soares, C.S.; da Silva, S.H.; Da Silva Filho, A.A.; Rodrigues, V. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 2009, 104, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.D.; Nascimento, C.; Lopes, P.O.; Nakano, E.; Yamaguchi, L.F.; Kato, M.J.; Kawano, T. Schistosoma mansoni: in vitro schistosomicidal activity of piplartine. Exp. Parasitol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Pica-Mattoccia, L.; Cioli, D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004, 34, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, L.; Bartlett, A.; Whitfield, P.J. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. J. Helminthol. 2002, 76, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Shuhua, X.; Binggui, S.; Chollet, J.; Tanner, M. Tegumental changes in adult Schistosoma mansoni harbored in mice treated with praziquantel enantiomers. Acta Trop. 2000, 76, 107–117. [Google Scholar] [CrossRef]
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008, 21, 659–667. [Google Scholar] [CrossRef] [PubMed]
- De Castro, H.G.; De Oliveira, L.O.; Barbosa, L.C.D.; Ferreira, F.A.; Da Silva, D.J.H.; Mosquim, P.R.; Nascimento, E.A. Content and composition of the essential oil of five accesses of mentrasto. Quim. Nova 2004, 27, 55–57. [Google Scholar]
- De Castro, H.G.; Barbosa, L.C.A.; Lui, J.J.; Oliveira, W.F.; Santos, G.R.; Carvalho, A.R.S. Growth, content and composition of the essential oil of accessions of mentrasto (Ageratum conyzoides) collected in the state of Tocantins, Brazil. Rev. Bras. Pl. Med. 2008, 10, 36–43. [Google Scholar]
- Sundufu, A.J.; Huang, S.S. Chemical composition of the essential oils of Ageratum conyzoides L. occurring in South China. Flavour Frag. J. 2004, 19, 6–8. [Google Scholar] [CrossRef]
- Wandji, J.; Bissangou, M.F.; Ouambra, J.M.; Silou, T.; Abena, A.A.; Keita, A. Essential oils of Ageratum conyzoides. Fitoterapia 1996, 67, 427–431. [Google Scholar]
- Smithers, S.R.; Terry, R.J. Infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of adult worms. Parasitology 1965, 55, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.H.; Keiser, J.; Chollet, J.; Utzinger, J.; Dong, Y.; Endriss, Y.; Vennerstrom, J.L.; Tanner, M. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Ch. 2007, 51, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Michaels, R.M.; Prata, A. Evolution and characteristics of Schistosoma mansoni eggs laid in vitro. J. Parasitol. 1968, 54, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Comley, J.C.W.; Rees, M.J.; Turner, C.H.; Jenkins, D.C. Calorimetric quantitation of filarial viability. Int. J. Parasitol. 1989, 19, 77–83. [Google Scholar] [CrossRef]
Sample Availability: Samples of the essential oil of Ageratum conyzoides, precocene I and (E) caryophyllene are available from the authors. |



| Group | Incubation period (h) | Number of separated worms (%) | Number of dead worms (%) | Decrease in motor activity | Number of worms with tegumental alteration | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slight (%) | Significant (%) | Partial (%) | Extensive (%) | |||||||||
| M | F | M | F | M | F | M | F | M | F | |||
| Controla | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| DMSO 1%b | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PZQc | 24 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | 25 | 25 | 50 | 50 |
| 10 μg/mL | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 50 μg/mL | 24 | 50 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 50 | 25 | 0 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 100 μg/mL | 24 | 50 | 25 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 |
| 120 | 75 | 100 | 75 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Compound | Retention time (min) | Retention index | Peak area (%) |
|---|---|---|---|
| α-Thujene | 4.909 | 924 | 0.16 |
| α-Pinene | 5.100 | 931 | 0.30 |
| β-Cubebene | 22.312 | 1384 | 0.62 |
| β-Elemene | 22.396 | 1386 | 0.42 |
| (E)-Caryophyllene | 23.594 | 1414 | 14.23 |
| α-Humulene | 25.059 | 1450 | 2.80 |
| Precocene I | 25.517 | 1461 | 74.30 |
| γ-Muurolene | 26.129 | 1476 | 3.44 |
| Germacrene D | 26.546 | 1486 | 0.59 |
| Bicyclogermacrene | 26.702 | 1490 | 3.14 |
| Total | 100.00 |
| Group | Incubation period (h) | Number of separated worms (%) | Number of dead worms (%) | Decrease in motor activity | Number of worms with tegumental alteration | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slight (%) | Significant (%) | Partial (%) | Extensive (%) | |||||||||
| M | F | M | F | M | F | M | F | M | F | |||
| Controla | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PZQ b 10 μM | 24 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | 25 | 25 | 50 | 50 |
| (3.1 μg/mL) | ||||||||||||
| (1) 25 μM | 24 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| (4.7 mg/mL) | 120 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| (1) 50 μM | 24 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (9.4 mg/mL) | 120 | 0 | 0 | 0 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| (1) 100 μM | 24 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| (18.8 mg/mL) | 120 | 0 | 0 | 0 | 75 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| (1) 200 μM | 24 | 50 | 0 | 0 | 75 | 75 | 0 | 0 | 0 | 0 | 0 | 0 |
| (37.6 mg/mL) | 120 | 50 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| (2) 25 μM | 24 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| (5.2 mg/mL) | 120 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| (2) 50 μM | 24 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| (10.4 mg/mL) | 120 | 0 | 0 | 0 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| (2) 100 μM | 24 | 0 | 0 | 0 | 50 | 75 | 0 | 0 | 0 | 0 | 0 | 0 |
| (20.8 mg/mL) | 120 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| (2) 200 μM | 24 | 0 | 0 | 0 | 75 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (41.6 mg/mL) | 120 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| (1+2) 25 μg/mL | 24 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (1+2) 50 μg/mL | 24 | 0 | 0 | 0 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 0 | 0 | 0 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (1+2) 100 μg/mL | 24 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (1+2) 200 μg/mL | 24 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| 120 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Melo, N.I.; Magalhaes, L.G.; De Carvalho, C.E.; Wakabayashi, K.A.L.; De P. Aguiar, G.; Ramos, R.C.; Mantovani, A.L.L.; Turatti, I.C.C.; Rodrigues, V.; Groppo, M.; et al. Schistosomicidal Activity of the Essential Oil of Ageratum conyzoides L. (Asteraceae) against Adult Schistosoma mansoni Worms. Molecules 2011, 16, 762-773. https://doi.org/10.3390/molecules16010762
De Melo NI, Magalhaes LG, De Carvalho CE, Wakabayashi KAL, De P. Aguiar G, Ramos RC, Mantovani ALL, Turatti ICC, Rodrigues V, Groppo M, et al. Schistosomicidal Activity of the Essential Oil of Ageratum conyzoides L. (Asteraceae) against Adult Schistosoma mansoni Worms. Molecules. 2011; 16(1):762-773. https://doi.org/10.3390/molecules16010762
Chicago/Turabian StyleDe Melo, Nathalya I., Lizandra G. Magalhaes, Carlos E. De Carvalho, Kamila A. L. Wakabayashi, Gabriela De P. Aguiar, Rafael C. Ramos, Andre L. L. Mantovani, Izabel C. C. Turatti, Vanderlei Rodrigues, Milton Groppo, and et al. 2011. "Schistosomicidal Activity of the Essential Oil of Ageratum conyzoides L. (Asteraceae) against Adult Schistosoma mansoni Worms" Molecules 16, no. 1: 762-773. https://doi.org/10.3390/molecules16010762