4. Experimental
4.1. General
Melting points were measured on a Büchi B-540 apparatus and are uncorrected. All
1H-NMR spectra were recorded on a 400 MHz spectrometer (Brüker AM). Chemical shifts were expressed as
δ values in parts in million (ppm) relative to tetramethylsilane (TMS). Mass spectral data were obtained on an Esquire-LC-00075 spectrometer. Reagents and solvents were purchased from common commercial suppliers and were used without further purification. Compounds
4a-c,
6c,
7c,
8a,b and
11c were prepared according to the procedures reported in previous references [
14,
17].
4.2. General Method for Synthesis of Prenylated Acetophenones 5
A solution of compound 4, prenyl bromide and potassium carbonate in DMF (100 mL) was heated at 100 °C under a N2 atmosphere for 6 h, and then the reaction mixture was poured into cold water and extracted with ethyl acetate. The organic phase was washed with brine and dried over anhydrous sodium sulfate. After removal of the solvent, the residue was dissolved in N,N-dimethylaniline (50 mL) and then heated at 190 °C for 3~5 h. The mixture was concentrated under vacuum, and the residue was purified by column chromatography on silica gel using (30/1, v/v) as eluent to give compounds 5.
2-Hydroxy-5-(3,3-dimethyl)allyl-4,6-dimethoxymethoxyacetophenone (5a): Reagents: compound 4a (10.0 g, 39.1 mmol), prenyl bromide (8.73 g, 58.6 mmol) and potassium carbonate (10.8 g, 78.2 mmol). Product: yellow oil (4.93 g, 39%); 1H-NMR (CDCl3, δ): 1.68 (s, 3H), 1.76 (s, 3H), 2.69 (s, 3H), 3.30 (d, 1H, J = 6.4 Hz), 3.45 (s, 3H), 3.51 (s, 3H), 4.95 (s, 2H), 5.14 (m, 1H), 5.21 (s, 2H), 6.46 (s, 1H), 12.93 (s, 1H, OH). ESI-MS: m/z [M-H]− 323.
2-Hydroxy-5-(3,3-dimethyl)allyl-4-dimethoxymethoxyacetophenone (5b): Reagents: compound 4b (10.0 g, 51.0 mmol), prenyl bromide (11.4 g, 76.5 mmol) and potassium carbonate (14.1 g, 102.0 mmol). Product: yellow oil (7.4 g, 55%). 1H-NMR (CDCl3, δ): 1.72 (s, 3H), 1.75 (s,3H), 2.55 (s, 3H), 3.25 (d, 2H, J = 7.2Hz), 3.47 (s, 3H), 5.23 (s, 2H), 5.26 (m, 1H), 6.60(s,1H), 7.43(s,1H), 12.54(s,1H). ESI-MS: m/z [M-H]− 263.
2-Hydroxy-3-(1,1-dimethyl)allyl-5-dimethoxymethoxyacetophenone (5c): Reagents: compound 4c (10.0 g, 51.0 mmol), prenyl bromide (11.4 g, 76.5 mmol) and potassium carbonate (14.1 g, 102.0 mmol). Product: yellow oil (6.19 g, 46%). 1H-NMR (CDCl3, δ): 1.44 (s, 3H), 1.47 (s, 3H), 2.55 (s, 3H), 3.48 (s, 3H), 4.85 (d, 1H, J = 17.2 Hz), 4.93 (d, 1H, J = 10.8 Hz), 5.24 (s, 2H), 6.05 (dd, 1H, J = 10.8, 17.2 Hz), 7.06 (d, 1H, J = 2.0 Hz), 7.33 (d, 1H, J = 2.0 Hz), 12.87 (s, 1H, OH). ESI-MS: m/z [M-H]− 263.
4.3. General Method for Synthesis of Prenylated Chalcones 6
To a cold solution of the acetophenone 5 and appropriate benzaldehyde in H2O-EtOH (1/4, v/v, 3 mL), 20% KOH in H2O-EtOH (1/4, v/v, 3 mL) was added with stirring. The resulting mixture was stirred under nitrogen at room temperature for 36 h, and then poured into ice-water (50 mL). The solution was acidified to pH ~ 2 with 1 N HCl, and extracted with ethyl acetate (20 mL × 3 times). The organic phase was washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuo. The residue was purified by column chromatography on silica gel using petroleum ether-ethyl acetate as eluant to give the desired compound 6.
2-Hydoxy-2'-chloro-4,6-dimethoxymethoxy-5-(3,3-dimethyl)allylchalcone (6a): Reagents: compound 5a (500.2 mg, 1.54 mmol), 2-chlorobenzaldehyde (227.8 mg, 1.62 mmol). Eluent: petroleum ether- ethyl acetate (20:1, v/v). Product: yellow oil (468.7 mg, 68%). 1H-NMR (CDCl3, δ): 1.68 (s, 3H,), 1.78 (s, 3H), 3.32 (d, 2H, J = 6.8 Hz), 3.43 (s, 3H), 3.49 (s, 3H), 5.21 (m, 1H), 5.25 (s, 2H), 5.26 (s, 2H), 6.39 (s, 1H,), 7.30 (m, 2H), 7.43 (m, 1H), 7.67 (m, 1H), 7.88 (d, 1H, J = 16.0 Hz), 8.12 (d, 1H, J = 16.0 Hz), 13.86 (s, 1H, OH). ESI-MS: m/z [M+H]+ 447.
2-Hydoxy-3',4'-dioxymethylene-4,6-dimethoxymethoxy-5-(3,3-dimethyl)allylchalcone (6b): Reagents: compound 5a (500.0 mg, 1.54 mmol), 3,4-dioxymethylenebenzaldehyde (243.1 mg, 1.62 mmol). Eluent: petroleum ether-ethyl acetate (18:1, v/v). Product: yellow oil (443.3 mg, 63%). 1H-NMR (CDCl3, δ): 1.70 (s, 3H,), 1.74 (s, 3H), 3.31 (d, 2H, J = 6.8 Hz), 3.50 (s, 3H), 3.54 (s, 3H), 5.21 (m, 1H), 5.24 (s, 2H), 5.28 (s, 2H), 6.01 (s, 2H), 6.43 (s, 1H), 6.83 (d, 1H, J = 7.6 Hz), 7.06 (dd, 1H, J = 7.6, 2.0 Hz), 7.08 (d, 1H, J = 2.0 Hz), 7.71 (d, 1H, J = 16.0 Hz), 7.78 (d, 1H, J = 16.0 Hz), 13.90 (s, 1H). ESI-MS: m/z [M+H]+ 457.
2-Hydoxy-4,4',6-trimethoxymethoxy-5-(3,3-dimethyl)allylchalcone (6d): Reagents: compound 5a (500.2 mg, 1.54 mmol), 3,4-dimethoxymethoxybenzaldehyde (269.1 mg, 1.62 mmol). Eluent: petroleum ether-ethyl acetate (18:1, v/v). Product: yellow oil (437.2 mg, 60%) . 1H-NMR (CDCl3, δ): 1.68 (s, 3H), 1.79 (s, 3H), 3.32 (d, 2H, J = 6.8 Hz), 3.47 (s, 3H), 3.52 (s, 3H), 3.53 (s, 3H), 5.21 (m, 1H), 5.22 (s, 2H), 5.23 (s, 2H), 5.25 (s, 2H), 6.42 (s, 1H,), 7.06 (d, 2H, J = 8.8 Hz), 7.55 (d, 2H, J = 8.8 Hz), 7.76 (d, 1H, J = 15.6 Hz), 7.85 (d, 1H, J = 15.6 Hz), 13.82 (s, 1H). ESI-MS: m/z [M+H]+ 473.
2-Hydoxy-3',4'-dioxymethylene-4-methoxymethoxy-5-(3,3-dimethyl)allylchalcone (6e): Reagents: compound 5b (500.3 mg, 1.90 mmol), 3,4-dimethoxymethoxybenzaldehyde (298.5 mg, 1.99 mmol). Eluent: petroleum ether-ethyl acetate (20:1, v/v). Product: yellow oil (540.3 mg, 72%). 1H-NMR (CDCl3, δ): 1.77 (s, 3H,), 1.78 (s, 3H), 3.31 (d, 2H, J = 6.8 Hz), 3.50 (s, 3H), 5.26 (s, 2H), 5.30 (m, 1H), 6.06 (s, 2H), 6.66 (s, 1H), 6.88 (d, 1H, J = 8.4 Hz), 7.15 (d, 1H, J = 8.4 Hz), 7.18 (d, 1H, J = 1.2 Hz), 7.40 (d, 1H, J = 16.0 Hz), 7.62 (s, 1H), 7.81 (d, 1H, J = 16.0 Hz), 13.30 (s, 1H, OH). ESI-MS: m/z [M+H]+ 397.
2-Hydoxy-3-(1,1-methyl)allyl-4'-methoxy-5-methoxymethoxychalcone (6f): Reagents: compound 5c (500.1 mg, 1.89 mmol), 4-methoxybenzaldehyde (270.5 mg, 1.99 mmol). Eluent: petroleum ether- ethyl acetate (25:1, v/v). Product: yellow oil (180.9 mg, 25%). 1H-NMR (CDCl3, δ): 1.52 (s, 3H), 1.57 (s, 3H), 3.52 (s, 3H), 3.86 (s, 3H), 5.01 (d, 1H, J = 18.0 Hz), 5.02 (d, 1H, J = 9.6 Hz), 5.15 (s, 2H), 6.26 (dd, J = 18.0, 9.6 Hz), 6.94 (d, 2H, J = 8.4 Hz), 7.25 (d, 1H, J = 2.0 Hz), 7.46 (d, 1H, J = 2.0 Hz), 7.47 (d, 1H, J = 15.6 Hz), 7.61 (d, 2H, J = 8.4 Hz), 7.88 (d, 1H, J = 15.6 Hz), 13.40 (s, 1H, OH). ESI-MS: m/z [M+H]+ 383.
2-Hydoxy-3-(1,1-methyl)allyl-4'-chloro-5-methoxymethoxychalcone (6g): Reagents: compound 5c (500 mg, 1.89 mmol), 4-chlorobenzaldehyde (279.4 mg, 1.99 mmol). Eluent: petroleum ether-ethyl acetate (25:1, v/v). Product: yellow oil (219.6 mg, 30%). 1H-NMR (CDCl3, δ): 1.52 (s, 3H), 1.55 (s, 3H), 3.51 (s, 3H), 5.01 (d, 1H, J = 17.2 Hz), 5.03 (d, 1H, J = 9.2 Hz), 5.15 (s, 2H), 6.26 (dd, J = 17.2, 9.2 Hz), 7.28 (d, 1H, J = 2.0 Hz), 7.40 (d, 2H, J = 8.4 Hz), 7.45 (d, 1H, J = 2.0 Hz), 7.55 (d, 1H, J = 15.6 Hz), 7.58 (d, 2H, J = 8.4 Hz), 7.83 (d, 1H, J = 15.6 Hz), 13.6 (s, 1H, OH). ESI-MS: m/z [M+H]+ 387.
4.4. General Method for Synthesis of Prenylated Flavanones 7
A solution of 6 and sodium acetate (500 mg, 6.10 mmol) in ethanol (5 mL) containing 3 drops of water was refluxed for 24 h. The mixture was poured into cold water (30 mL) and extracted with ethyl acetate (10 mL × 3 times). The organic phase was washed with brine, dried over sodium sulfate. After removing the solvent, the residue was purified by column chromatography on silica gel using petroleum ether-ethyl acetate as eluent to give 7.
2'-Chloro-5,7-dimethoxymethoxy-6-(3,3-dimethyl)allylflavanone (7a): Reagents: compound 6a (300 mg, 0.67 mmol); Eluent: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (162 mg, 54%). 1H-NMR (CDCl3, δ): 1.66 (s, 3H), 1.69 (s, 3H), 2.75 (dd, 1H, J = 16.4 Hz, 2.8 Hz), 2.93 (dd, 1H, J = 13.4, 16.4 Hz), 3.36 (d, 2H, J = 6.8 Hz), 3.48 (s, 3H), 3.52 (s, 3H), 5.17 (m, 1H), 5.20 (s, 2H), 5.23 (s, 2H), 5.68 (dd, 1H, J = 2.8, 13.4 Hz), 6.65 (s, 1H), 7.30 (td, 1H, J = 2.0, 7.6 Hz), 7.36 (td, 1H, J = 2.0, 7.6 Hz), 7.39 (dd, 1H, J =2.0, 7.6 Hz), 7.20 ( dd, 1H, J =2.0, 7.6 Hz). ESI-MS: m/z [M+H]+ 447.
3',4'-Dioxymethylene-5,7-dimethoxymethoxy-6-(3,3-dimethyl)allylflavanone (7b): Reagents: compound 6b (300.2 mg, 0.66 mmol). Eluent: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (204.1 mg, 68%). 1H-NMR (CDCl3, δ): 1.71 (s, 3H), 1.73 (s, 3H), 2.76 (dd, 1H, J = 16.8 Hz, 2.8 Hz), 2.98 (dd, 1H, J = 12.8, 16.4 Hz), 3.27 (d, 2H, J = 7.2 Hz), 3.42 (s, 3H), 3.47 (s, 3H), 5.22 (s, 2H), 5.26 (m, 1H), 5.27 (s, 2H), 5.34 (dd, J = 2.8, 12.8Hz), 5.99 (s, 2H), 6.69 (s, 1H), 6.83 (d, 1H, J = 8.0 Hz), 6.90 (dd, 1H, J = 2.0, 8.0), 6.98 (d, 1H, J = 2.0 Hz), 7.69 (s, 1H). ESI-MS: m/z [M+H]+ 457.
4',5,7-Trimethoxymethoxy-6-(3,3-dimethyl)allylflavanone (7d): Reagent: compound 6d (300.5 mg, 0.64 mmol). Eluent: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (156.3 mg, 52%). 1H-NMR (CDCl3, δ): 1.68 (s, 3H), 1.70 (s, 3H), 2.82 (dd, 1H, J = 16.4 Hz, 2.8 Hz), 2.98 (dd, 1H, J =13.4, 16.4 Hz), 3.33 (d, 2H, J = 7.2 Hz), 3.48 (s, 3H), 3.50 (s, 3H), 3.54 (s, 3H), 5.20 (m, 1H), 5.22 (s, 2H), 5.23 (s, 2H), 5.26 (s, 2H), 5.37 (dd, 1H, J = 2.8 Hz, 13.4 Hz), 6.68 (s, 1H), 7.10 (d, 2H, J = 8.0 Hz), 7.39 (d, 2H, J = 8.0 Hz). ESI-MS: m/z [M+H]+ 473.
3',4'-Dioxymethylene-5-methoxymethoxy-6-(3,3-dimethyl)allylflavanone (7e): Reagents: compound 6e (300.2 mg, 0.76 mmol). Elent: petroleum ether-ethyl acetate (12:1, v/v). Product: pale yellow syrup (180.1 mg, 60%). 1H-NMR (CDCl3, δ): 1.71 (s, 3H), 1.73 (s, 3H), 2.76 (dd, 1H, J = 16.8 Hz, 2.8 Hz), 2.98 (dd, 1H, J =12.8, 16.4 Hz), 3.27 (d, 2H, J = 7.2 Hz), 3.47 (s, 3H), 5.22 (s, 2H), 5.26 (m, 1H), 5.34 (dd, J = 2.8, 12.8Hz), 5.99 (s, 2H), 6.69 (s, 1H), 6.83 (d, 1H, J = 8.0 Hz), 6.90 (dd, 1H, J = 2.0, 8.0), 6.98 (d, 1H, J = 2.0 Hz), 7.69 (s, 1H). ESI-MS: m/z [M+H]+ 397.
4'-Methoxy-6-methoxymethoxy-8-(1,1-dimethyl)allyl-flavanone (7f): Reagent: compound 6f (300.0 mg, 0.79 mmol). Elu-nt: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (150 mg, 50%). 1H-NMR (CDCl3, δ): 1.41 (s, 3H), 1.45 (s, 3H), 2.85 (dd, J = 2.0, 16.8 Hz, 1H), 3.02 (dd, J = 13.2, 16.8 Hz, 1H), 3.48 (s, 3H), 3.92 (s, 3H), 4.94 (d, 1H, J = 17.2 Hz), 4.95 (d, 1H, J = 10.8 Hz), 5.21 (s, 2H), 5.24 (dd, J = 2.0, 13.2 Hz), 6.09 (dd, 1H, J = 10.8, 17.2 Hz), 7.01 (d, 2H, J = 8.4 Hz), 7.12 (d, 1H, J = 2.4 Hz), 7.38 (d, 1H, J = 2.4 Hz), 7.40 (d, 2H, J = 8.4 Hz). ESI-MS: m/z [M+H]+ 383.
4'-Chloro-6-methoxymethoxy-8-(1,1-dimethyl)allyl-flavanone (7g): Reagents: compound 6g (300.3 mg, 0.78 mmol). Eluent: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (129.1 mg, 43%). 1H-NMR (CDCl3 δ): 1.42 (s, 3H), 1.48 (s, 3H), 2.83 (dd, 1H, J = 2.0, 16.8 Hz), 3.95 (dd, 1H, J = 13.2, 16.8 Hz), 3.44 (s, 3H), 4.90 (d, 1H, J = 17.2 Hz), 4.98 (d, 1H, J = 10.8 Hz), 5.25 (s, 2H), 5.32 (dd, 1H, J = 2.0, 13.2 Hz), 6.03 (dd, 1H, J = 10.8, 17.2 Hz), 7.13 (d, 1H, J = 2.4 Hz), 7.39 (d, 1H, J = 2.4 Hz), 7.44 (brd, 4H). ESI-MS: m/z [M+H]+ 387.
4.5. General Method for Synthesis of Compounds 10
A solution of compound 8, 2-chloro-diethylethanamine hydrochloride and potassium carbonate in acetone (5 mL) was refluxed for 10 h. After cooling to room temperature, the mixture was filtered and the solution was concentrated in vacuo. The residue was purified by column chromatography on silica gel using petroleum ether-ethyl acetate as eluant (3:1, v/v) to give 9, which was further dissolved in 5 mL 3N HCl/methanol (1/4, v/v) and refluxed for 1 h. Then, the reaction mixture was evaporated in vacuo to give yellow solid and washed with ether to afford 10.
2-(2-(Diethylamino)ethoxy-3'-bromo-4,6-dimethoxymethoxychalcone (10a): Reagents: compound 8a (200 mg, 0.47 mmol), 2-chloro-N,N-diethylethanamine hydrochlororide (122 mg, 0.71 mmol) and potassium carbonate (194.6 mg, 1.41 mmol). Product: yellow solid (167.3 mg, 72%), m.p. 180–182 °C. 1H-NMR (MeOH-d4, δ): 1.08 (d, 1H, J = 6.8 Hz), 3.10 (m, 4H), 3.48 (m, 2H), 4.38 (brs, 2H), 6.03 (s, 1H), 6.07 (s, 1H), 7.40 (d, 1H, J = 8.0 Hz), 7.51 (d, 1H, J = 16.0 Hz), 7.63 (d, 1H, J = 8.0 Hz ), 7.65 (d, 1H, J = 16.0 Hz), 7.78 (d, 1H, J = 8.0 Hz), 8.01 (s, 1H), 10.06 (s, 1H, OH), 10.37 (s, 1H, OH), 12.48 (s, 1H, OH). ESI-MS: m/z [M+H]+ 434.
2-(2-(Diethylamino)ethoxy-3',4'-dioxymethylene-4,6-dimethoxymethoxy-chalcone (10b): Reagents: compound 8b (201 mg, 0.52 mmol), 2-chloro-N,N-diethylethanamine hydrochlororide (133.7 mg, 0.78 mmol) and potassium carbonate (215.3 mg, 1.56 mmol). Product: yellow solid (161.8 mg, 78%), m.p. 227–230 °C. 1H-NMR (MeOH-d4, δ): 1.10 (d, 1H, J = 6.8 Hz), 3.10 (m, 4H), 3.49 (m, 2H), 4.39 (brs, 2H), 6.02 (s, 1H), 6.06 (s, 1H), 6.10 (s, 2H), 6.97 (d, 1H, J = 8.0 Hz), 7.25 (d, 1H, J = 8.0 Hz), 7.46 (s, 1H), 7.45 (d, 1H, J = 16.0 Hz), 7.51 (d, 1H, J = 16.0 Hz), 10.07 (s, 1H, OH), 10.55 (s, 1H, OH), 12.55 (s, 1H, OH). ESI-MS: m/z [M+H]+ 400.
4.6. General Method for Synthesis of Compounds 11
A solution of 7 in 3N HCl/methanol (1/4, v/v, 3 mL) was refluxed for 2 h, then poured into cold water (15 mL) and extracted with ethyl acetate (5 mL × 3 times). The organic phase was washed with brine and then dried over anhydrous sodium sulfate. After removal of the solvent, the residue was purified by column chromatography on silica gel using petroleum ether-ethyl acetate as eluant to give 11.
2'-Chloro-5,7-dihydroxy-6-(3,3-dimethyallyl)flavanone (11a): Reagents: compound 11a (150 mg, 0.34 mmol). Eluent: petroleum ether-ethyl acetate (6:1, v/v). Product: white solid (90.3 mg, 75%), m.p. 130–133 °C. 1H-NMR (acetone-d6, δ): 1.59 (s, 3H), 1.69 (s, 3H), 2.78 (dd, 1H, J = 2.8, 17.2 Hz), 3.07 (dd, 1H, J = 12.8, 17.2 Hz), 3.12 (d, 2H, J = 6.8 Hz), 5.17 (m, 1H), 5.79 (dd, 1H, J = 2.8, 12.8 Hz), 5.99 (s, 1H), 7.39–7.48 (m, 3H), 7.77 (dd, 1H, J =1.6, 7.6 Hz), 9.66 (s, 1H), 12.35 (s, 1H). ESI-MS: m/z [M+H]+ 359.
3',4'-Dioxymethylene-5,7-dihydroxy-6-(3,3-dimethyl)allylflavanone (11b): Reagents: compound 11b (150 mg, 0.33 mmol). Eluent: petroleum ether-ethyl acetate (6:1, v/v). Product: white solid (79.9 mg, 68%), m.p. 156–158 °C. 1H-NMR (acetone-d6, δ): 1.59 (s, 3H), 1.68 (s, 3H), 2.69 (dd, J =3.2, 16.8 Hz, 1H), 3.10 (dd, 1H, J = 12.8, 16.8 Hz), 3.20 (d, 2H, J = 7.2 Hz), 5.18 (m, 1H), 5.39 (dd, 1H, J = 12.8 Hz, 3.2 Hz), 5.98 (s, 2H), 6.00 (s, 1H), 6.83 (d, 1H, J = 8.0 Hz), 6.97 (d, J = 8.0 Hz), 7.04 (s, 1H), 9.53 (s, 1H), 12.39 (s, 1H). ESI-MS: m/z [M+H]+ 369.
2-(4'-Hydroxyphenyl)-5-hydroxy-6,7-dihydro-8,8-dimethyl-4H,8H-benzo[1,2-b;5,4-b']dipyran-4-one (11d): Reagents: compound 11d (150 mg, 0.32 mmol). Eluent: petroleum ether-ethyl acetate (6:1, v/v). Product: white solid (41.1 mg, 38%), m.p. 122–125 °C. 1H-NMR (DMSO-d6, δ): 1.33 (s, 6H), 1.82 (t, 2H, J = 7.2 Hz), 2.58 (t, 2H, J = 7.2 Hz), 2.73 (dd, 1H, J = 2.8, 17.2 Hz), 3.16 (dd, 1H, J = 12.8, 17.2 Hz), 5.42 (dd, 1H, J = 2.8, 12.8 Hz), 5.84 (s, 1H), 6.89 (d, 1H, J = 8.0 Hz), 7.38 (dd, 1H, J = 8.0 Hz), 8.51 (1H, OH), 12.57 (1H, OH). ESI-MS: m/z [M+H]+ 341.
2-(3,4-Dioxymethylenephenyl)-6,7-dihydro-8,8-dimethyl-4H,8H-benzo[1,2-b;5,4-b']dipyran-4-one (11e): Reagents: compound 11e (150 mg, 0.38 mmol). Eluent: petroleum ether: ethyl acetate (6:1, v/v). Product: white solid (60 mg, 45%), m.p. 95–98 °C .1H-NMR (DMSO-d6, δ): 1.34 (s, 3H), 1.35 (s, 3H), 1.81 (t, 1H, J = 6.8 Hz), 2.76 (t, 1H, J = 6.8 Hz), 2.77 (dd, 1H, J = 2.4, 17.2 Hz), 2.98 (dd, 1H, J = 12.8, 17.2 Hz), 5.32 (dd, 1H, J = 2.4, 12.8 Hz), 5.97 (s, 2H), 6.38 (s, 1H), 6.82 (d, 1H, J = 7.6 Hz), 6.90 (dd, 1H, J = 2.0, 7.6 Hz), 6.97 (d, 1H 1H, J = 2.0 Hz), 7.26 (s, 1H), 7.67 (s, 1H). ESI-MS: m/z [M+H]+ 353.
4'-Methoxy-6-hydroxy-8-(1,1-dimethyl)allyl-flavanone (11f): Reagents: compound 11f (150 mg, 0.39 mmol). Eluent: petroleum ether: ethyl acetate (10:1, v/v). Product: yellow syrup (55.7, 42%). 1H-NMR (CDCl3 δ): 1.43 (s, 3H), 1.44 (s, 3H), 2.82 (dd, J = 2.0, 16.8 Hz, 1H), 3.00 (dd, J = 13.2, 16.8 Hz, 1H), 3.84 (s, 3H), 4.89 (d, 1H, J = 17.2 Hz), 4.95 (d, 1H, J = 10.8 Hz), 4.97 (s, 1H, OH), 5.30 (dd, J = 2.0, 13.2 Hz), 6.11 (dd, 1H, J = 10.8, 17.2 Hz), 6.95 (d, 2H, J = 8.4 Hz), 7.11 (d, 1H, J = 2.4 Hz), 7.27 (d, 1H, J = 2.4 Hz), 7.40 (d, 2H, J = 8.4 Hz). ESI-MS: m/z [M+H]+ 339.
4'-Chloro-6-hydroxy-8-(1,1-dimethyl)allyl-flavanone (11g): Reagents: compound 11g (100 mg, 0.26 mmol). Eluent: petroleum ether-ethyl acetate (10:1, v/v). Product: yellow solid (29.2 mg, 33%), m.p. 90–92 °C. 1H-NMR (CDCl3 δ): 1.42 (s, 3H), 1.45 (s, 3H), 2.83 (dd, 1H, J = 2.0, 16.8 Hz), 3.92 (dd, 1H, J = 13.2, 16.8 Hz), 4.89 (d, 1H, J = 17.2 Hz), 4.95 (d, 1H, J = 10.8 Hz), 5.00 (s, 1H, OH), 5.30 (dd, 1H, J = 2.0, 13.2 Hz), 6.09 (dd, 1H, J = 10.8, 17.2 Hz), 7.13 (d, 1H, J = 2.4 Hz), 7.36 (d, 1H, J = 2.4 H), 7.41 (brd, 4H). ESI-MS: m/z [M+H]+ 343.
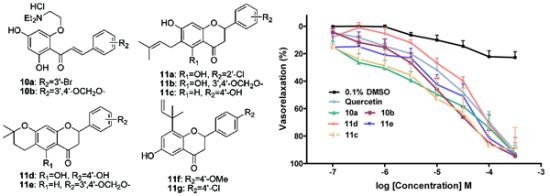
4.7. Vasorelaxant Activities Assay
Vascular rings were prepared from the aorta of male Male Sprague-Dawley rats (four to six months old and weighing on average 250 g), and contraction studies were performed following the general procedure detailed in the literature [
18]. After an equilibration period of at least 1 h, isometric contractions induced by PE (1 μM) were obtained. When contraction of the tissue in response to this vasoconstrictor agent had stabilized (after about 20 min), cumulatively increasing concentrations of the tested compounds were added to the bath at 15~20 min intervals (the time needed to obtain steady-state relaxation). Control tissues were simultaneously subjected to the same procedures, but omitting the compounds and adding the vehicle. The flavonoids-induced maximal relaxation (
Emax) in aortic rings was calculated as a percentage of the contraction in response to PE (1 μM). The half maximum effective concentration (EC
50) was defined as the concentration of the flavonoids that induced 50% of maximum relaxation from the contraction elicited by PE (1 μM) and was calculated from the concentration response curve by nonlinear regression (curve fit) using GraphPad Prism (Version 4.0).
4.8. Computational Methods
4.8.1. Data Preparation
For the development of 2D-QSAR model, five newly synthesized compounds and thirty previous compounds [
14,
15,
16,
19] with available EC
50 were selected. They were divided into training set (26 molecules) and test set (nine molecules) considering both structural diversity and wide coverage of biological activity ranges. The structures of all compounds were optimized in Discovery Studio 2.0 software (Accelrys, Inc. San Diego, CA, USA). The resulted geometry of molecules were inputted into Dragon software [
20], which can calculate constitutional descriptors, topological descriptors, walk and path counts, information indices, 2D autocorrelations, edge adjacency indices, Burden eigenvalue descriptors, Burden eigenvalue descriptors,
etc. After the calculation of the molecular descriptors, those that stayed constant for all molecules were eliminated, and pairs of variables with a correlation coefficient greater than 0.75 were classified as intercorrelated with one of each correlated pair was deleted.
4.8.2. Enhanced Replacement Method-Multiple Linear Regressions (ERM-MLR)
Enhanced Replacement Method (ERM) proposed by Mercader
et al. [
21] is a modified version of Replacement Method (RM) [
22,
23]. The purpose of both algorithms is to choose an optimal subset of d (d < D) descriptors from the pool of D descriptors with minimum standard deviation SD:
where N is the number of molecules in the training set, and
resi the residual for molecule
i (difference between the experimental and predicted property). Considering that SD(d
n) is a distribution on a discrete space of D!/d!(D–d) disordered points d
n, ERM produces linear models that are quite similar with the full search (FS) but with much less computational work. First, an initial set of descriptors
dk was selected at random. And one of the descriptors, say
Xki, with all the remaining descriptors (D-d) was replaced by other descriptor, one by one, and the set with the smallest value of SD was kept. Second, from the resulting set the descriptor with the greatest SD in its coefficient is chosen and all the remaining D-d descriptors, one by one, until the set remains unmodified. More detailed information about these algorithms can be found in reference [
21].
4.8.3. Leave-One-Out Cross Validation
Validation of the models was required to test the predictive ability and generalization of the methods by cross validation as well as test set prediction. The leave-one-out cross validation, a special case of the cross-validation technique [
24] was employed to find the promising QSAR model. Given n samples available in a data set and m candidate models, each model is trained with n − 1 samples and then is tested on the sample that was left out. This process is repeated n times until every sample in the data set have been used once as a cross-validation instance. Finally, cross validation correlation coefficient (
Qloo2) of LOO-CV, a measure of the model generalization capability, for all candidate models can be obtained as below:
where
yi is the desired output and
![Molecules 16 08257 i004]()
is the actual output of the model, and
n is the number of compounds in the analyzed set.








 is the actual output of the model, and n is the number of compounds in the analyzed set.
is the actual output of the model, and n is the number of compounds in the analyzed set.