Antihyperlipidemic Properties of Novel N-(Benzoylphenyl)-5-substituted-1H-indole-2-carboxamides in Triton WR-1339-Induced Hyperlipidemic Rats
Abstract
:1. Introduction



2. Results and Discussion
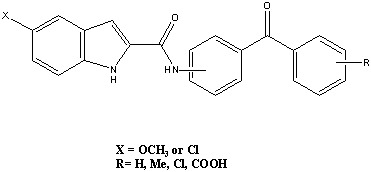
2.1. Chemical Studies
2.2. Pharmacological Studies
2.2.1. Induction of Hyperlipidemia by Triton WR-1339
| Lipid profile | TC (mg/dl) | TG (mg/dl) | HDL-C(mg/dl) | LDL-C (mg/dl) |
|---|---|---|---|---|
| NCG | 107 ± 0.76 | 131 ± 1.52 | 50.3 ± 0.40 | 31.1 ± 0.47 |
| HG | 228 ± 3.32 b | 1526 ± 8.06 b | 30.6 ± 0.92 b | 61.6 ± 0.88 b |
| C8 | 227 ± 3.53 | 1544 ± 3.38 | 28.0 ± 0.73 | 65.9 ± 0.50 |
| C9 | 112 ± 1.09 a | 85 ± 1.60 b | 59.2 ± 0.88 b | 23.5 ± 0.53 a |
| C15 | 220 ± 4.63 | 1566 ± 4.44 | 29.9 ± 0.33 | 63.6 ± 0.42 |
| C16 | 109 ± 2.10 a | 96 ± 0.70 b | 61.0 ± 0.56 b | 24.9 ± 0.23 a |
| C18 | 106 ± 0.99 a | 103 ± 2.43 b | 63.1 ± 0.31 b | 21.2 ± 0.31 a |
| BF | 221 ± 2.40 | 132 ± 0.64 b | 48.5 ± 0.48 b | 60.9 ± 0.79 |
2.2.2. Effect of Compounds 8, 9, 15, 16, 18 and Bezafibrate on Rat Plasma Lipid Profile
3. Experimental
3.1. General
3.2. Animals and Treatments
3.3. Triton Model of Hyperlipidemia
3.4. Pharmacological Experimental Design
3.5. Statistical Analysis
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References
- Epstein, F.M. The hepatorenal syndrome-newer perspectives. N. Engl. J. Med. 1992, 327, 1774–1778. [Google Scholar]
- Raasch, R.H. Hyperlipidemias in Applied Therapeutics. In The Clinical Use of Drugs; Young, L.Y., Koda-Kimble, M.A., Eds.; Edwards Brothers: Ann Arbor, MI, USA, 1988; pp. 1743–1745. [Google Scholar]
- Frishman, W.H. Biologic markers as predictors of cardiovascular disease. Am. J. Med. 1998, 104, 18S–27S. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Hazzard, W.R.; Schrott, H.G.; Bierman, E.L.; Motulsky, A.G. Hyperlipidemia in coronary heart disease I. Lipid levels in 500 survivors of myocardial infarction. J. Clin. Invest. 1973, 52, 1533–1543. [Google Scholar] [CrossRef]
- Martin, M.J.; Hulley, S.B.; Browner, W.S.; Kuller, L.H.; Wentworth, D. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet 1986, 2, 933–936. [Google Scholar]
- West, K.M.; Ahuja, M.S.; Bennet, P.H. The role of circulating glucose and triglyceride concentrations and their interactions with other "risk factors" as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care 1983, 6, 361–369. [Google Scholar]
- Kellner, A.; Correll, J.W.; Ladd, A.T. Sustained hyperlipemia induced in rabbits by means of intravenously injected surface-active agents. J. Exp. Med. 1951, 93, 373–384. [Google Scholar] [CrossRef]
- Schotz, M.C.; Scanu, A.; Page, I.H. Effect of Triton on lipoprotein lipase of rat plasma. Am. J. Physiol. 1957, 188, 399–402. [Google Scholar]
- Kalopissis, A.D.; Griglio, S.; Malewiak, M.I.; Rosen, R. Effect of a high-fat diet on rat very low density lipoprotein secretion. Biochim. Biophys. Acta 1980, 620, 111–119. [Google Scholar] [CrossRef]
- Paoletti, R. Comparative studies on hypocholesterolemic agents. Am. J. Clin. Nutr. 1962, 10, 277–284. [Google Scholar]
- Da Rocha, J.T.; Sperança, A.; Nogueira, C.W.; Zeni, G. Hypolipidaemic activity of orally administered diphenyl diselenide in triton WR-1339-induced hyperlipidaemia in mice. J. Pharm. Pharmacol. 2009, 61, 1673–1679. [Google Scholar]
- Frick, M.H.; Elo, O.; Haapa, K.; Heinonen, O.P.; Heinsalmi, P.; Helo, P.; Huttunen, J.K.; Kaitaniemi, P.; Koskinen, P.; Manninen, V.; Mäenpää, H.; Mälkönen, M.; Mänttäri, M.; Norola, S.; Pasternack, A.; Pikkarainen, J.; Romo, M.; Sjöblom, T.; Nikkilä, E.A. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N. Engl. J. Med. 1987, 317, 1237–1245. [Google Scholar]
- Schoonjans, K.; Staels, B.; Auwerx, J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 1996, 37, 907–925. [Google Scholar]
- Bosies, E.; Heerdt, R.; Kuknle, H.F.; Schmidt, F.H.; Stach, H. Hypoglycemically and Hypolipidemically Active Derivatives of Phenylalkane Carboxylic Acids. U.S. Patent 4238506 A, 19801209, 1980; CAN 95:150207, 1980.
- Dasseux, J.L.H.; Oniciu, C.D. Ketone Compounds and Compositions for Cholesterol Management and Related Uses. WO 2002030860 A2 20020418, 2002; U.S. Patent 136:325529, 2002.
- Kopin, A.S.; Carey, M.; Wang, D. Methods of Altering Intestinal Motility and Absorption of Hydrophobic Compounds through the Use of Agonists and/or Antagonists of the Cholecystokinin–1 Receptor. U.S. Patent Appl. Publ. US 20060177438 A1, 20060810, 2006, Appllication number US 2005-53553, 2005; CAN 145:224869, 2005.
- Sher, P.M.; Ellsworth, B.A. Triglyceride and Triglyceride–Like Prodrugs of Glycogen Phosphorylase Inhibiting Compounds. U.S. Patent Appl. Publ. US 20040142938 A1, 20040722, 2004; US 7098235 B2, 2006; U.S. CAN 141:140474, 2004.
- Shahwan, M.; Shattat, G.; Al-Qirim, T.; Abu Sheikha, G.; Al-Hiari, Y.; El-Huneidi, W.; Jarab, A.; AL-Najdawi, M. Synthesis and pharmacological evaluation of novel substitutedand unsubstituted N-(benzoylphenyl)-1H-indole-2-carboxamides as potent antihypertriglyceridemic agents. Z. Naturforsch. 2010, 65c, 309–316. [Google Scholar]
- Shattat, G.; Al-Qirim, R.; Al-Hiari, Y.; Abu Sheikha, G.; Al-Qirim, T.; El-Huneidi, W.; Shahwan, M. Synthesis and Anti-Hyperlipidemic Evaluation of N-(Benzoylphenyl)-5-fluoro-1H-indole-2-carboxamide Derivatives in Triton WR-1339-Induced Hyperlipidemic Rats. Molecules 2010, 15, 5840–5849. [Google Scholar] [CrossRef]
- Al-Qirim, T.; Shahwan, M.; Shattat, G.; Al-Hiari, Y.; Abu Sheikha, G.; Zaidi, S. Pharmacological evaluation of novel indole-2-carboxamides as potent lipid-lowering agents in Triton-WR1339-induced hyperlipidemic rats. Z. Naturforsch. 2009, 64c, 619–625. [Google Scholar]
- Pérez, C.; Canal, J.R.; Campello, J.E.; Adelaida, R.; Torres, M.D. Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytother. Res. 1999, 13, 188–191. [Google Scholar] [CrossRef]
- Khanna, A.K.; Chauder, R.; Chandan, S.; Srivastava, A.K.; Kapoor, N.K. Hypolipidemic activity of Achyranthus aspera linn in normal and triton induces htperlipemic rats. Indian J. Exp. Biol. 1992, 30, 128–130. [Google Scholar]
- Khanna, A.K.; Rizvi, F.; Chander, R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J. Ethnopharmacol. 2002, 82, 19–22. [Google Scholar] [CrossRef]
- Schurr, P.E.; Schultz, J.R.; Parkinson, T.M. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids 1972, 7, 69–74. [Google Scholar]
- Otway, S.; Robinson, D.S. The effect of the nonionic detergent (Triton) on the removal of triglyceride fatty acids from the blood of the rats. J. Physiol. 1967, 190, 309–319. [Google Scholar]
- Malloy, M.J.; Kan, J.P. Medical management of hyperlipidemic states. Ad. Int. Med 1994, 39, 603–631. [Google Scholar]
- Anila, L.; Vijayalakshmi, N.R. Flavonoids from Emblica officinalis and Mangifera indica- effectiveness for dyslipidemia. J. Ethnopharmacol. 2002, 79, 81–87. [Google Scholar] [CrossRef]
- Vijaya, C.; Ramanathan, M.; Suresh, B. Lipid lowering activity of ethanolic extract of leaves of Aegle marmelos (Linn.) in hyperlipidaemic models of Wistar albino Rats. Indian J. Exp. Biol. 2009, 47, 182–185. [Google Scholar]
- Nagao, K.; Sakono, M.; Nakayama, M.; Hirakawa, T.; Imaizumi, K. Effect of gemfibrozil on triacylglycerol synthesis and secretion by liver and lipoprotein lipase activity in adipose tissue of rats. Comp. Biochem. Phys. B 1999, 124, 289–294. [Google Scholar] [CrossRef]
- Nakajima, T.; Tanaka, N.; Kanbe, H.; Hara, A.; Kamijo, Y.; Zhang, X.; Gonzalez, F.J.; Aoyama, T. Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: A novel peroxisome proliferator-activated receptor alpha-independent mechanism. Mol. Pharmacol. 2009, 75, 782–792. [Google Scholar] [CrossRef]
- Mori, Y.; Tokutate, Y.; Oana, F.; Matsuzawa, A.; Akahane, S.; Tajima, N. Bezafibrate-induced changes over time in the expression of uncoupling protein (UCP) mRNA in the tissues: A study in spontaneously type 2 diabetic rats with visceral obesity. J. Atheroscler. Thromb. 2004, 11, 224–231. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Hiari, Y.; Shattat, G.; Al-Qirim, T.; El-Huneidi, W.; Sheikha, G.A.; Hikmat, S. Antihyperlipidemic Properties of Novel N-(Benzoylphenyl)-5-substituted-1H-indole-2-carboxamides in Triton WR-1339-Induced Hyperlipidemic Rats. Molecules 2011, 16, 8292-8304. https://doi.org/10.3390/molecules16108292
Al-Hiari Y, Shattat G, Al-Qirim T, El-Huneidi W, Sheikha GA, Hikmat S. Antihyperlipidemic Properties of Novel N-(Benzoylphenyl)-5-substituted-1H-indole-2-carboxamides in Triton WR-1339-Induced Hyperlipidemic Rats. Molecules. 2011; 16(10):8292-8304. https://doi.org/10.3390/molecules16108292
Chicago/Turabian StyleAl-Hiari, Yusuf, Ghassan Shattat, Tariq Al-Qirim, Waseem El-Huneidi, Ghassan Abu Sheikha, and Suhair Hikmat. 2011. "Antihyperlipidemic Properties of Novel N-(Benzoylphenyl)-5-substituted-1H-indole-2-carboxamides in Triton WR-1339-Induced Hyperlipidemic Rats" Molecules 16, no. 10: 8292-8304. https://doi.org/10.3390/molecules16108292