Synthesis, Metal Ion Complexation and Computational Studies of Thio Oxocrown Ethers
Abstract
:1. Introduction
2. Results and Discussion

2.1. Computational Results
| Thiocrown ethers | Cation | Ke (1:1) | LogKe(1:1) | -ΔGθ1:1 |
|---|---|---|---|---|
 | Na+ | 159005,00 | 5,201411 | 7091,71 |
| K+ | 4659,09 | 3,668301 | 5001,44 | |
| Ca2+ | 21002,62 | 4,322273 | 5893,08 | |
| Zn2+ | 4134,76 | 3,61645 | 4930,72 | |
| Mg2+ | 22862,18 | 4,359118 | 5943,31 | |
| Ag+ | 15602,02 | 4,193181 | 5717,07 | |
| Fe2+ | 63856,70 | 4,805206 | 6551,52 | |
 | Na+ | 24556,00 | 4,390158 | 5985,63 |
| K+ | 1177370,00 | 6,070913 | 8277,21 | |
| Ca2+ | 1334785,00 | 6,125411 | 8351,51 | |
| Zn2+ | 9608,34 | 3,982648 | 5430,02 | |
| Mg2+ | 113650,30 | 5,055571 | 6892,87 | |
| Ag+ | 6486,80 | 3,812031 | 5197,40 | |
| Fe2+ | 2542753,00 | 6,405304 | 8733,12 | |
 | Na+ | 128719,00 | 5,109643 | 6966,59 |
| K+ | 1073250,00 | 6,030701 | 8222,38 | |
| Ca2+ | 516059,00 | 5,712699 | 7788,81 | |
| Zn2+ | 66872,94 | 4,82525 | 6578,85 | |
| Mg2+ | - | - | - | |
| Ag+ | 15602,07 | 4,193182 | 5717,07 | |
| Fe2+ | 30157,18 | 4,479391 | 6107,29 | |
 | Na+ | 136555,00 | 5,135308 | 7001,58 |
| K+ | 217,76 | 2,337978 | 3187,65 | |
| Ca2+ | 24444,83 | 4,388187 | 5982,94 | |
| Zn2+ | 58836,12 | 4,769644 | 6503,03 | |
| Mg2+ | - | - | - | |
| Ag+ | 246,23 | 2,391341 | 3260,40 | |
| Fe2+ | 3701,47 | 3,568374 | 4865,20 | |
 | Na+ | 4144,79 | 3,617503 | 4932,18 |
| K+ | 57052,90 | 4,756278 | 6484,81 | |
| Ca2+ | 37945,99 | 4,579166 | 6243,33 | |
| Zn2+ | 31015,46 | 4,491578 | 6123,91 | |
| Mg2+ | 22825,90 | 4,358428 | 5942,37 | |
| Ag+ | 54208,9 | 4,734071 | 6454,529 | |
| Fe2+ | 390.0 | 2,591065 | 3532,71 | |
 | Na+ | 127313,00 | 5,104873 | 6960,09 |
| K+ | 22056,90 | 4,343544 | 5922,08 | |
| Ca2+ | 37945,99 | 4,579166 | 6243,33 | |
| Zn2+ | 11118,95 | 4,046064 | 5516,49 | |
| Mg2+ | 517167,30 | 5,713631 | 7790,08 | |
| Ag+ | 298,72 | 2,475264 | 3374,83 | |
| Fe2+ | 6359,96 | 3,803454 | 5185,71 |
| HOMO | LUMO | ΔE | |
|---|---|---|---|
| B1 | 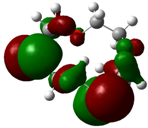 | 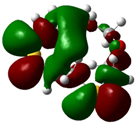 | |
| E (eV) | −0.219 | 0.030 | 0.249 |
| B2 | 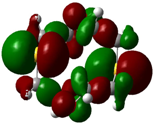 | 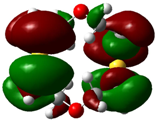 | |
| E (eV) | −0.209 | 0.042 | 0.251 |
| B3 | 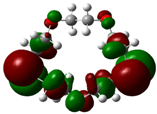 |  | |
| E (eV) | −0.228 | 0.024 | 0.249 |
| B4 |  | 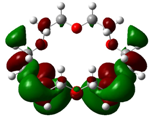 | |
| E (eV) | −0.202 | 0.053 | 0.255 |
| B5 |  | 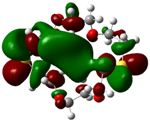 | |
| E (eV) | −0.217 | 0.031 | 0.248 |
| B6 |  |  | |
| E (eV) | −0.229 | 0.019 | 0.248 |
| Complex | Charges on Oxygen atoms | Charges on Sulfur atoms |
|---|---|---|
| B1 | −0.591 / −0.599 | 0.196 / 0.199 |
| B2 | −0.591 / −0.591 | 0.186 / 0.186 |
| B3 | −0.578 / −0.579 / −0.579 | 0.207 / 0.207 |
| B4 | −0.573 / −0.573 / −0.576 / −0.583 | 0.227 / 0.227 |
| B5 | −0.580 / −0.581 / −0.584 / −0.600 | 0.201 / 0.211 |
| B6 | −0.575 / −0.580 / −0.580 / −0.582 / −0.582 | 0.210 / 0.214 |
3. Experimental
3.1. General
3.1.1. The Synthesis of Dithiols
3.1.2. The Synthesis of Oxo-thiocrown Ethers
3.2. Conductimetric Method
4. Conclusions
Acknowledgments
- Sample Availa1bility: Samples of all compounds are available from the authors.
References and Notes
- Melson, G.A. Coordination Chemistry of Macrocyclic Compounds; Plenum Publishing Corp.: New York, NY, USA, 1979. [Google Scholar]
- Formanowskii, A.A.; Mikhura, I.V. Synthesis of Macrocyclic Compounds. In Macrocyclic Compounds in Analytical Chemistry; Zolotov, Y.A., Ed.; Wiley- Interscience: New York, NY, USA, 1997; pp. 5–39. [Google Scholar]
- Chartres, J.D.; Davies, M.S.; Lindoy, L.F.; Meehan, G.V.; Wei, G. Macrocyclic ligand design: The interaction of selected transition and post-transition metal ions with a 14-membered N2S2-donor macrocycle. Inorg. Chem. Commun. 2006, 9, 751–754. [Google Scholar] [CrossRef]
- Groth, A.M.; Lindoy, L.F.; Meehan, G.V. New linked macrocyclic systems derived from selectively protected S2N2 macrocycles. J. Chem. Soc. Perkin Trans. 1996, 1, 1553–1558. [Google Scholar]
- Lindoy, L.F.; Baldwin, D.S. Ligand design for selective metal-ion transport through liquid membranes. Pure Appl. Chem. 1989, 61, 909–914. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakamura, M.; Ikeda, T.; Ikeda, K.; Ando, H.; Shibutani, Y.; Yajima, S.; Kimura, K. Synthesis and metal-ion binding properties of monoazathiacrown ethers. J. Org. Chem. 2001, 66, 7008–7012. [Google Scholar]
- Alp, H.; Gok, H.Z.; Kantekin, H.; Ocak, Ü. Synthesis and metal ion binding properties of thiaaza crown macrocycles. J. Hazard. Mater. 2008, 159, 519–522. [Google Scholar] [CrossRef]
- Bernardo, M.M.; Heeg, M.J.; Schroeder, R.R.; Ochrymowycz, L. Comparison of the influence of saturated nitrogen and sulfur donor atoms on the properties of copper(II/I)-macrocyclic polyamino polythiaether ligand complexes: Redox potentials and protonation and stability constants of CuIL species and new structural data. Inorg. Chem. 1992, 31, 191–198. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; McAuley, A. Synthesis of an N2S-cyclodecane macrocycle and its nickel(II) complex. J. Chem. Soc. Dalton Trans. 1992, 2967–2970. [Google Scholar] [CrossRef]
- Blake, A.J.; Reid, G.; Schroder, M. Synthesis and electrochemistry of nickel and cobalt complexes of mixed thia-aza crown ethers: single-crystal structures of [Ni([18]aneN2S4)][PF6]2·0.33H2O and [CO([18]aneN2S4)][PF6]3·3H2O ([18]aneN2S4=1,4,10,13-tetrathia-7,16-diazacyclooctadecane). J. Chem. Soc. Dalton Trans. 1994, 3291–3297. [Google Scholar]
- Dalley, N.K.; Larson, S.B.; Smith, J.S.; Matheson, K.L.; Izatt, R.M.; Christensen, J.J. The crystal structures of 10-oxa-1,4,7-trithiacyclododecane, 7,10,13-trioxa-1,4-dithiacyclopentadecane, 7,10,13,16-tetraoxa-1,4-dithiacyclooctadecane and 4,7,13,16-tetraoxa-1,10-dithiacycloocta-decane. J. Heterocycl. Chem. 1981, 18, 463–467. [Google Scholar] [CrossRef]
- Adhikary, B.; Lucas, C.R. Copper(II) Complexes of N2S3 Ligands Involving Aromatic Nitrogen and Thioether Donors and Having High Redox Potentials. Inorg. Chem. 1994, 33, 1376–1381. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and kinetic data for macrocycle interaction with cations, anions, and neutral molecules. Chem. Rev. 1995, 95, 2529–2586. [Google Scholar] [CrossRef]
- Lucas, C.R.; Liang, W.; Miller, D.O.; Bridson, J.N. Metal complexes of 1-oxa-4,7-dithiacyclononane. Inorg. Chem. 1997, 36, 4508–4513. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1965, 89, 7017–7036. [Google Scholar] [CrossRef]
- Pedersen, C.J.; Frensdorff, H.K. Macrocyclic polyethers and their complexes. Angew. Chem. Int. Ed. Eng. 1972, 11, 16. [Google Scholar] [CrossRef]
- Vendilo, A.G.; Djigailo, D.I.; Smirnova, S.V.; Torocheshnikova, I.I.; Popov, K.I.; Krasovsky, V.G.; Pletnev, I.V. 18-Crown-6 and Dibenzo-18-crown-6 Assisted Extraction of Cesium from Water into Room Temperature Ionic Liquids and Its Correlation with Stability Constants for Cesium Complexes. Molecules 2009, 14, 5001–5016. [Google Scholar] [CrossRef]
- Hogen-Esch, T.E.; Smid, J. Conductivities and thermodynamics of dissociation of fluorenyl salts and their complexes with dimethyl-dibenzo-18-crown-6. J. Phys. Chem. 1975, 79, 233–238. [Google Scholar] [CrossRef]
- Hopkins, H.P.; Norman, A.B. Conductance and infrared studies on acetonitrile solutions containing crown ethers and alkali metal salts. J. Phys. Chem. 1980, 84, 309–314. [Google Scholar] [CrossRef]
- Evans, D.F.; Wellington, S.L.; Nads, J.A.; Cussler, E.L. The conductance of cyclic polyether-cation complexes. J. Solution Chem. 1972, 1, 499–506. [Google Scholar] [CrossRef]
- Çiçek, B.; Çakır, Ü.; Erk, Ç. The determination of crown-cation complexation behavior in dioxane/water mixtures by conductometric studies. Polym. Adv. Tecnol. 1998, 9, 831–836. [Google Scholar] [CrossRef]
- Rossotti, F.J.C.; Rossotti, H. The Determination of Stability Constants and Other Equilibrium Constants in Solution; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1961. [Google Scholar]
- Inoue, Y.; Hakushi, T.; Liv, Y. Action binding by macrocycles. In Conductometric Behavior of Cation-Macrocycle Complexes in Solutions; Takeda, Y., Ed.; Marcel Dekker: New York, NY, USA, 1990; pp. 133–178. [Google Scholar]
- Cicek, B.; Cakir, U.; Azizoglu, A. The associations of macrocyclic ethers with cations in 1,4-dioxane/ water mixtures; potentiometric Na+ and K+ binding measurements and computational study. J. Incl. Phenom. Macrocycl. Chem. 2011. [Google Scholar] [CrossRef]
- Lind, J.E.; Zwolnikand, J.J.; Fuoss, R.M. Calibration of conductance cells at 25° with aqueous solutions of potassium Chloride. J. Am. Chem. Soc. 1959, 81, 1557–1559. [Google Scholar] [CrossRef]
- Ugras, H.I.; Cakir, U.; Azizoglu, A.; Kılıc, T.; Erk, C. Experimental, theoretical and biological activity study on the acyl substituted benzo-18-crown-6, dibenzo-18-crown-6, and dibenzo-24-crown-8. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 159–165. [Google Scholar] [CrossRef]
- Toman, P.; Makrlik, E.; Vanura, P. A combined experimental and theoretical study on the complexation of the ammonium ion with benzo-18-crown-6. Monatsh. Chem. 2010, 141, 301–304. [Google Scholar] [CrossRef]
- Hancock, R. Molecular mechanics calculations and metal recognation. Acc. Chem. Res. 1990, 23, 253–257. [Google Scholar] [CrossRef]
- Shargh, D.N.; Hosseini, M.M.; Ohaninan, T. Configurational and Conformational Properties of 1,3,7,9-Tetraphospha-Cyclododeca-1,2,7,8- tetraene: An Ab Initio Study and NBO Analysis. Phosphorus Sulfur. 2008, 183, 2410–2420. [Google Scholar] [CrossRef]
- Azizoglu, A.; Yildiz, C.B. Ring-opening mechanism of lithium bromosilacyclopropylidenoids to silaallenes. Organometallics 2010, 29, 6739–6743. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X.; Yi, S.; Wang, N.; Peng, Y. DFT study of the carbon- and nitrogen-pivot lariat crown ethers and their complexes with alkali metal cations: Na+, K+. J. Comp. Chem. 2009, 30, 2674–2683. [Google Scholar] [CrossRef]
- Yuan, M.; Xueye, W.; Xin, J.; Ling, Y.; Cuihuan, R. DFT study for a seria of less-symmetrical crown ethers and their complexes with alkali metal cations. Chin. J. Chem. 2010, 28, 1835–1843. [Google Scholar] [CrossRef]
- Makrlik, E.; Toman, P.; Vanura, P. A combined extraction and DFT study on the complexation of the silver cation with dibenzo-18-crown-6. Monatsh. Chem. 2011, 142, 137–140. [Google Scholar] [CrossRef]
- Didier, S.; Gaudel-Siri, A.; Pons, J.M.; Liotard, D.; Rajzmann, M. Reaction mechanism studies made simple using simulated annealing. Potential energy surface exploration. J. Mol. Struct. (Theochem) 2002, 588, 71–78. [Google Scholar] [CrossRef]
- Khohlova, S.S.; Lebedev, N.G.; Bondarev, S.L.; Knyukshto, V.N.; Turban, A.A.; Mikhailova, V.A.; Ivanov, A.I. Electronic structure of laser dye DCM and its derivatives. Int. J. Quant. Chem. 2005, 104, 189–196. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, version C02; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Azizoglu, A.; Demirkol, O.; Kilic, T.; Yildiz, Y.K. Incorporation of an allene unit into 1,4-dihydronapthalene: Generation of 1,2-benzo-1,4,5-cycloheptatriene and its dimerization. Tetrahedron 2007, 63, 2409–2413. [Google Scholar] [CrossRef]
- Kilbas, B.; Azizoglu, A.; Balci, M. Edo- and exo-configured cyclopropylidenes incorporated into the norbornadiene skeleton: Generation, rearrangement to allenes, and the effect of remote substituents on carbene stability. J. Org. Chem. 2009, 74, 7075–7083. [Google Scholar] [CrossRef]
- Kurtaran, R.; Odabaşıoğlu, S.; Azizoğlu, A.; Kara, H.; Atakol, O. Experimental and computational study on [2,6-bis(3,5-dimethyl-n-pyrazoyl)pyridine]- (dithiocyanato)mercury (II). Polyhedron 2007, 26, 5069–5074. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation- energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar]
- Fukui, K.; Yonezawa, T.; Shingu, H. A molecular orbital theory of reactivity in aromatic hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley: London, UK, 1976. [Google Scholar]
- Azizoglu, A. Quantum chemical ınvestigation of monostanna[n]cyclacenes. Struct. Chem. 2003, 14, 575–580. [Google Scholar] [CrossRef]
- Odabasioglu, S.; Kurtaran, R.; Azizoglu, A.; Kara, H.; Oz, S.; Atakol, O. Experimental and computational study on [2, 6-bis(3, 5-dimethyl-N-pyrazolyl) pyridine]-(dithiocyanato)mercury (II). Cent. Eur. J. Chem. 2009, 7, 402–409. [Google Scholar] [CrossRef]
- Turker, L.; Azizoglu, A. The Effect of boron substitution on cyclacenes. J. Mol. Struct. (Theochem) 2001, 535, 151–157. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Çiçek, B.; Yıldız, A. Synthesis, Metal Ion Complexation and Computational Studies of Thio Oxocrown Ethers. Molecules 2011, 16, 8670-8683. https://doi.org/10.3390/molecules16108670
Çiçek B, Yıldız A. Synthesis, Metal Ion Complexation and Computational Studies of Thio Oxocrown Ethers. Molecules. 2011; 16(10):8670-8683. https://doi.org/10.3390/molecules16108670
Chicago/Turabian StyleÇiçek, Baki, and Ahmet Yıldız. 2011. "Synthesis, Metal Ion Complexation and Computational Studies of Thio Oxocrown Ethers" Molecules 16, no. 10: 8670-8683. https://doi.org/10.3390/molecules16108670




