Alkaloids from Marine Ascidians
Abstract
:1. Introduction
2. Pyridoacridine Alkaloids


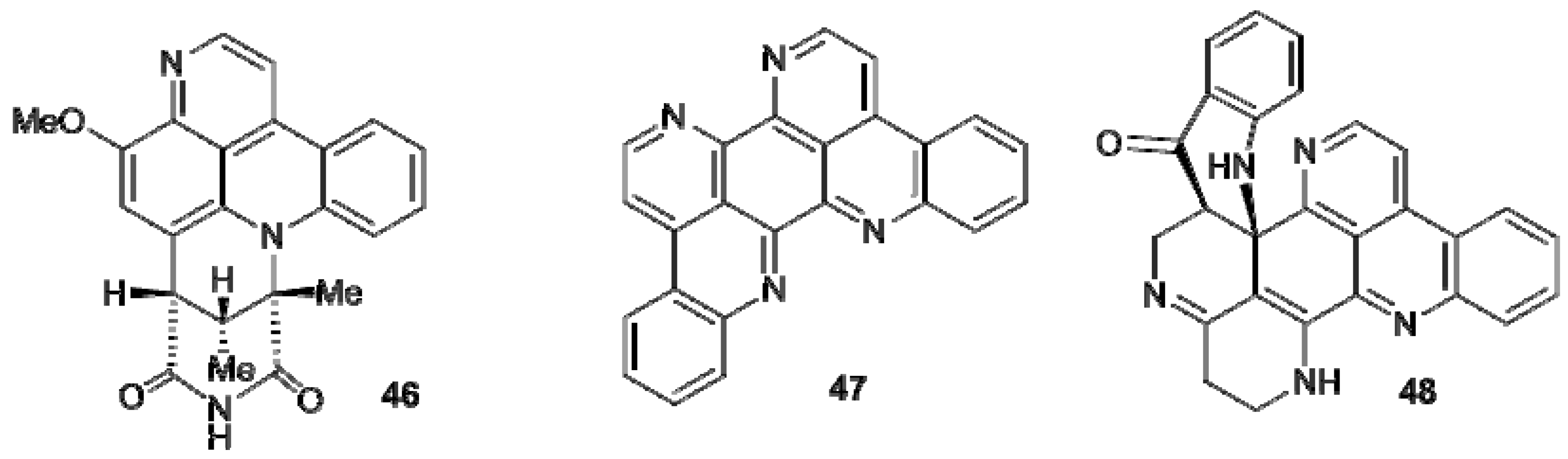
3. Carboline-Based Alkaloids
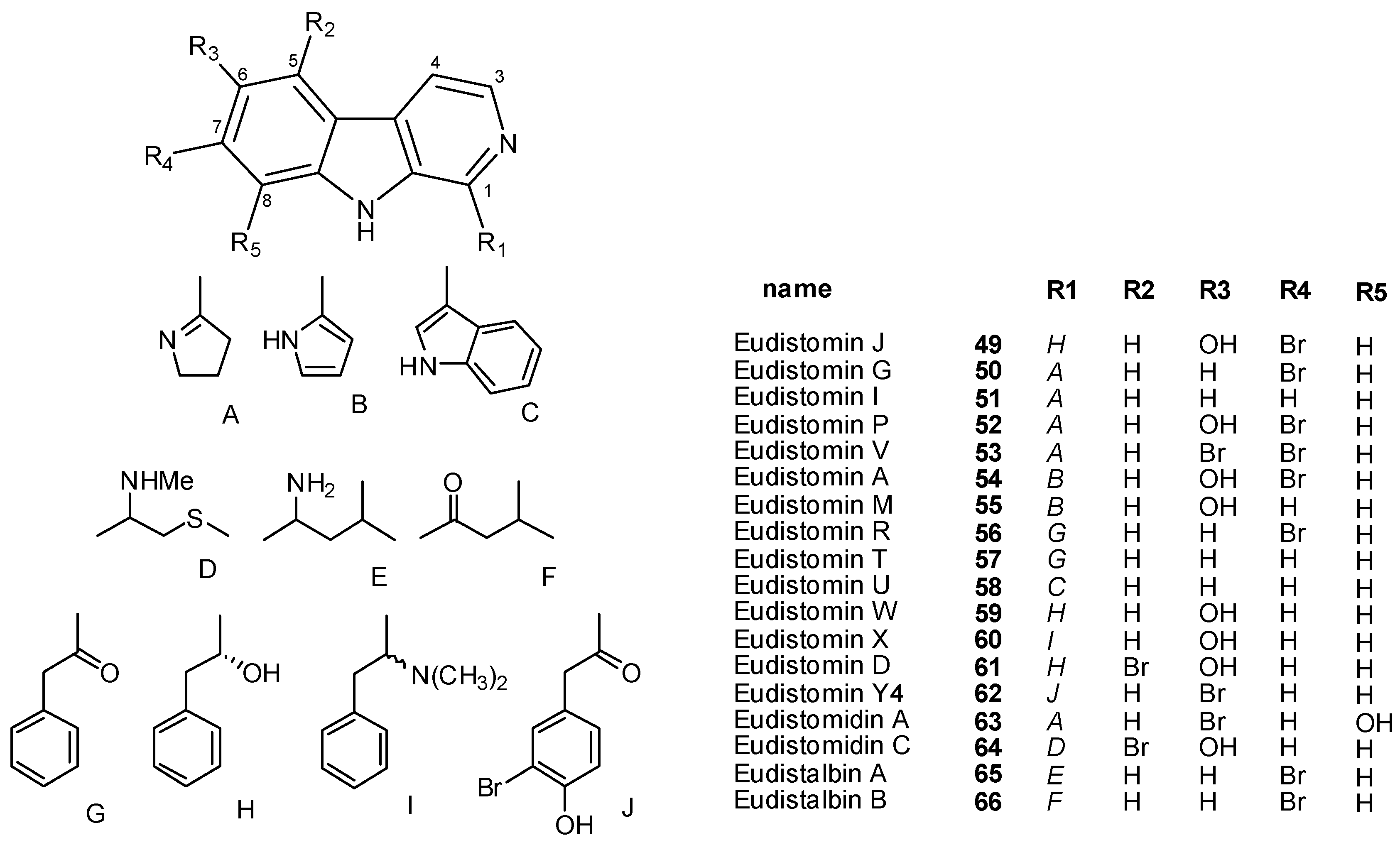
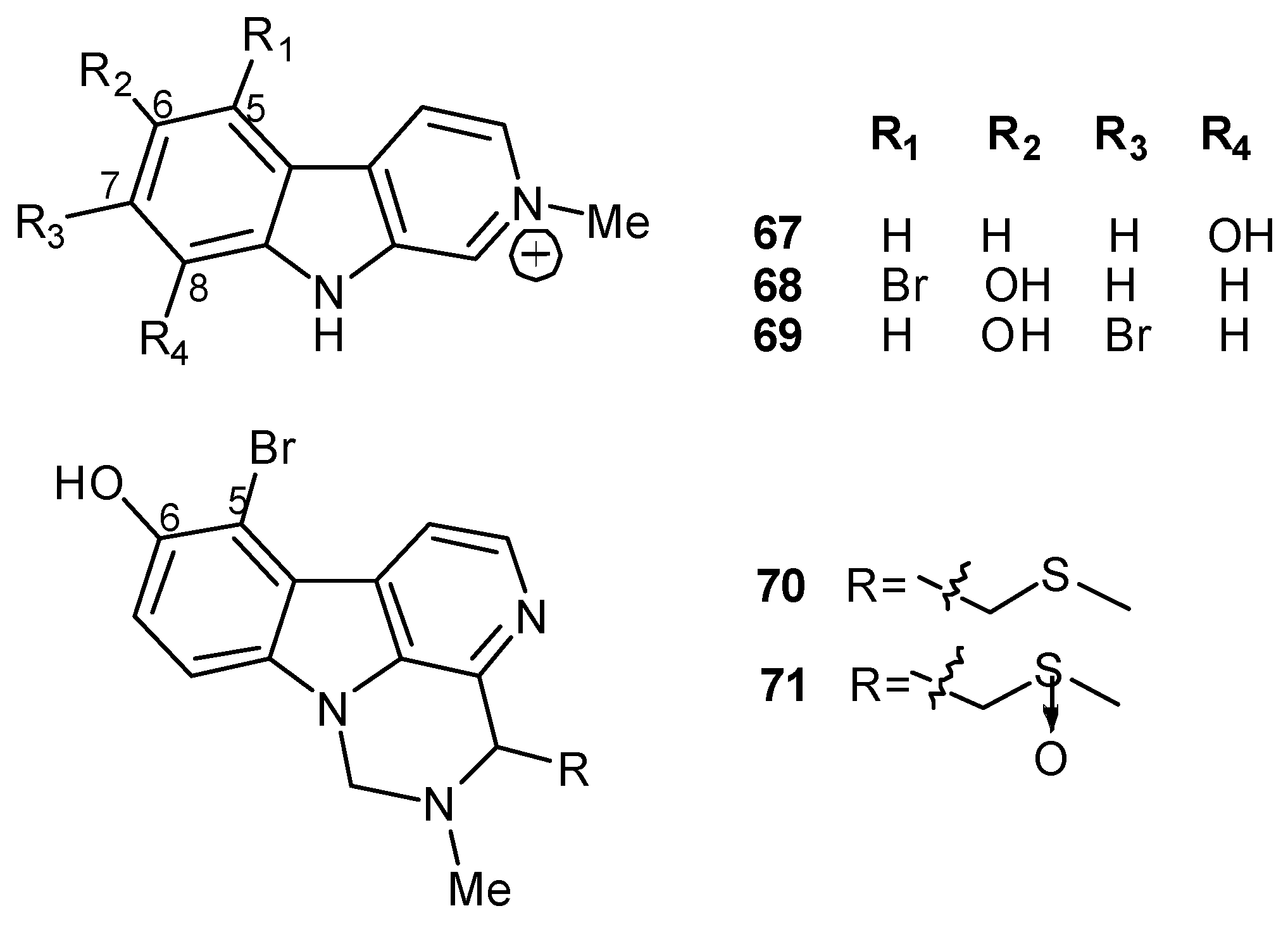




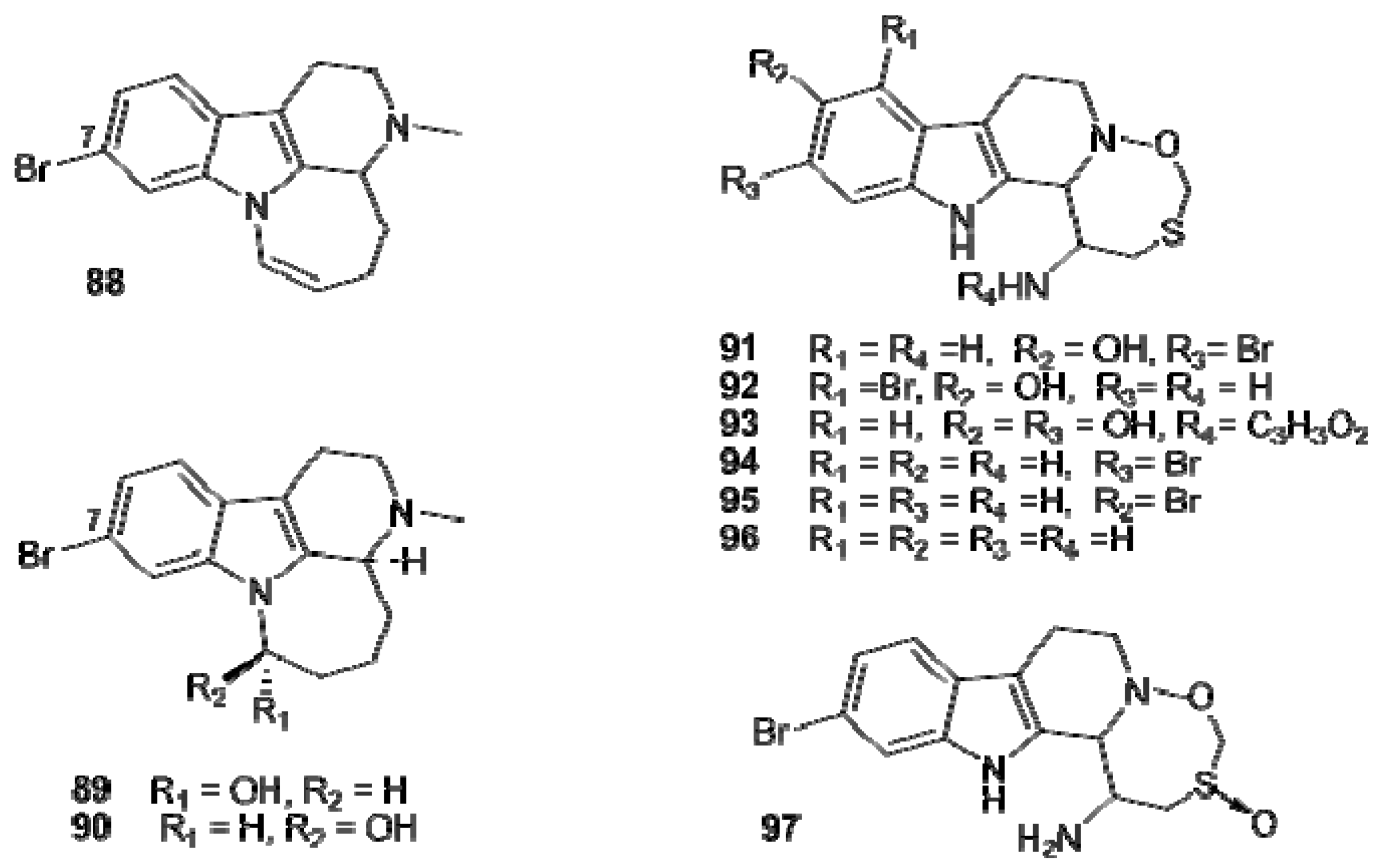


4. Indole-Based Alkaloids





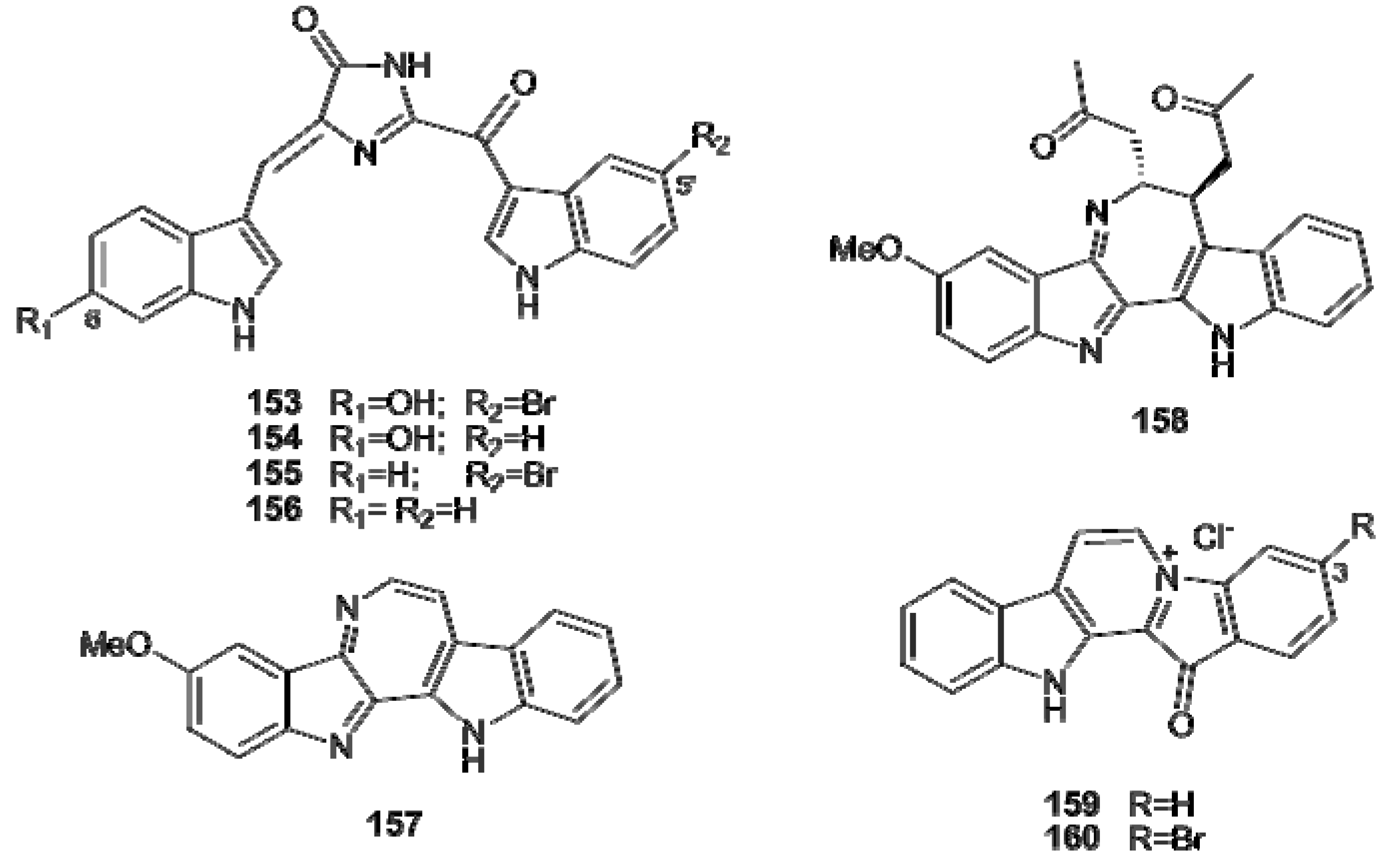
5. Tyrosine- and Phenylalanine-Derived Alkaloids


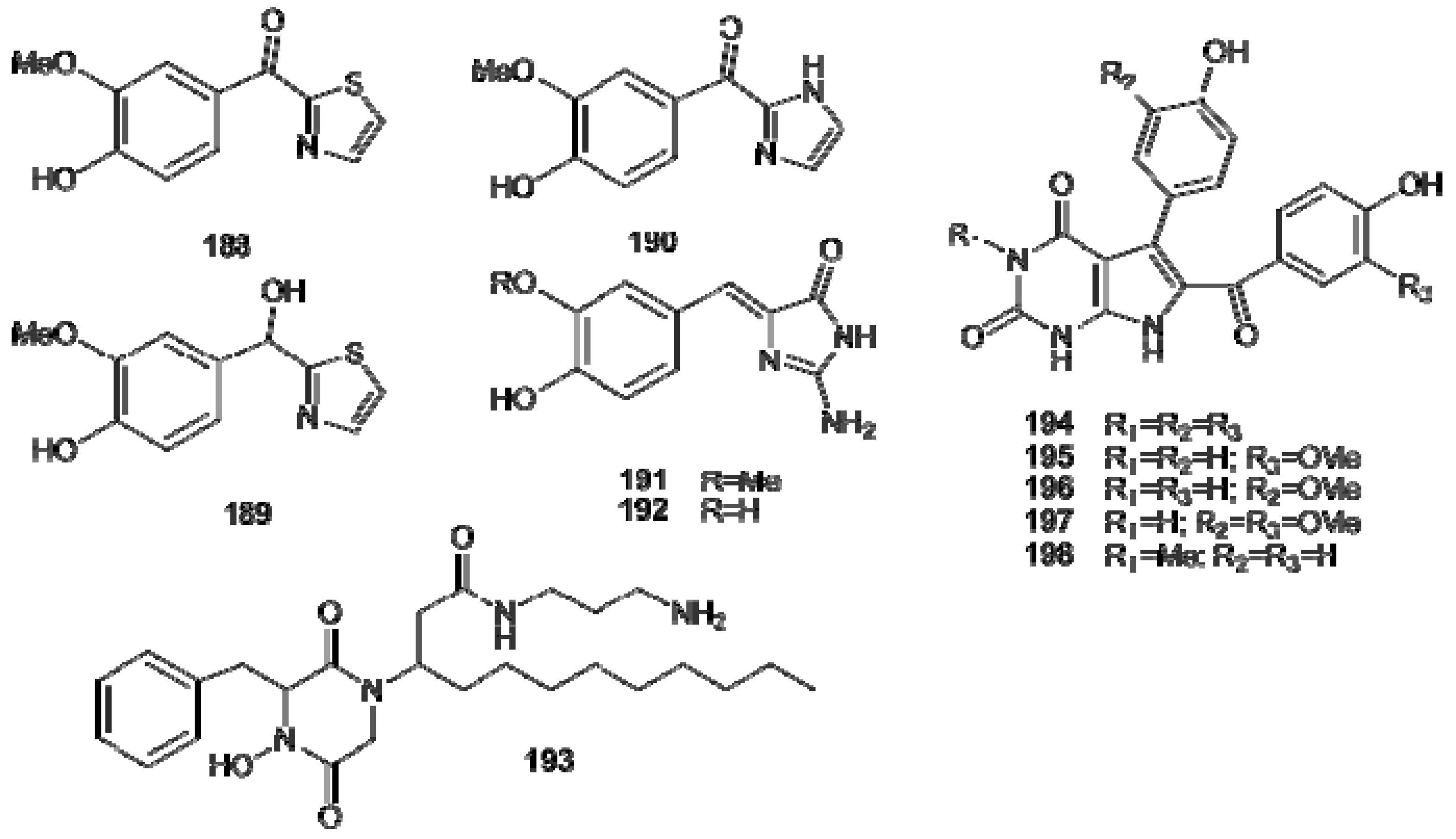





6. Lysine-Derived Alkaloids
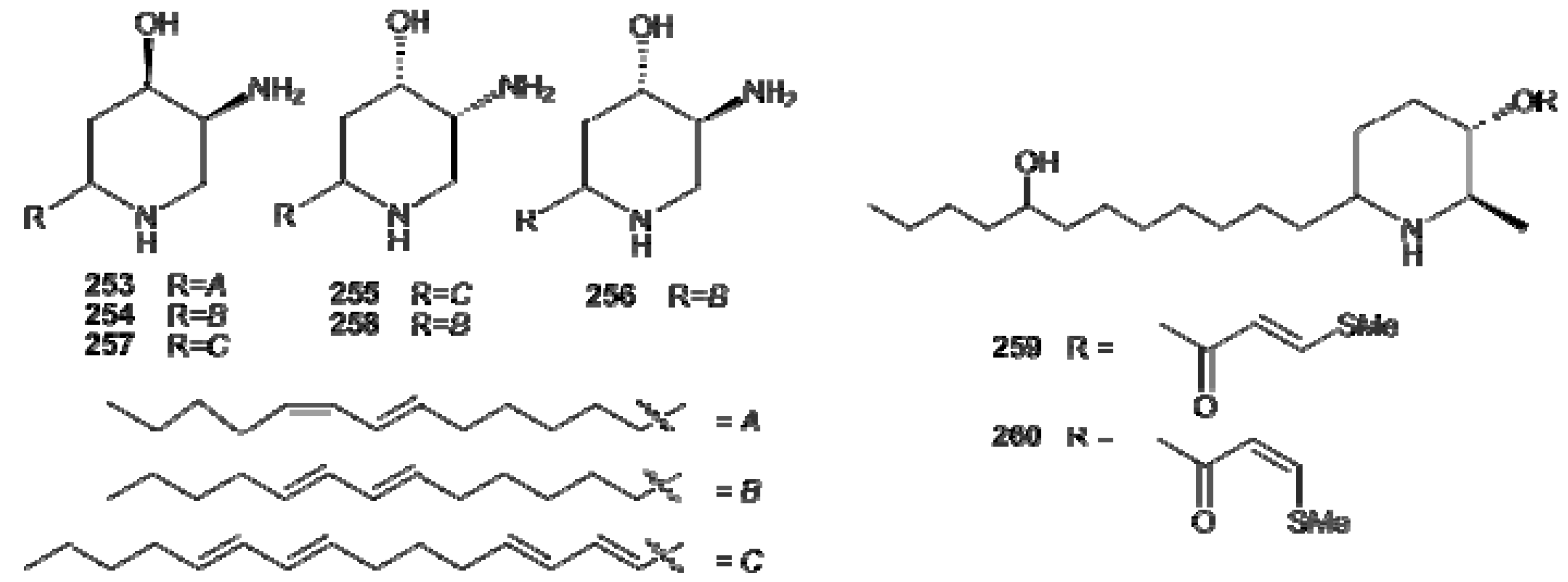
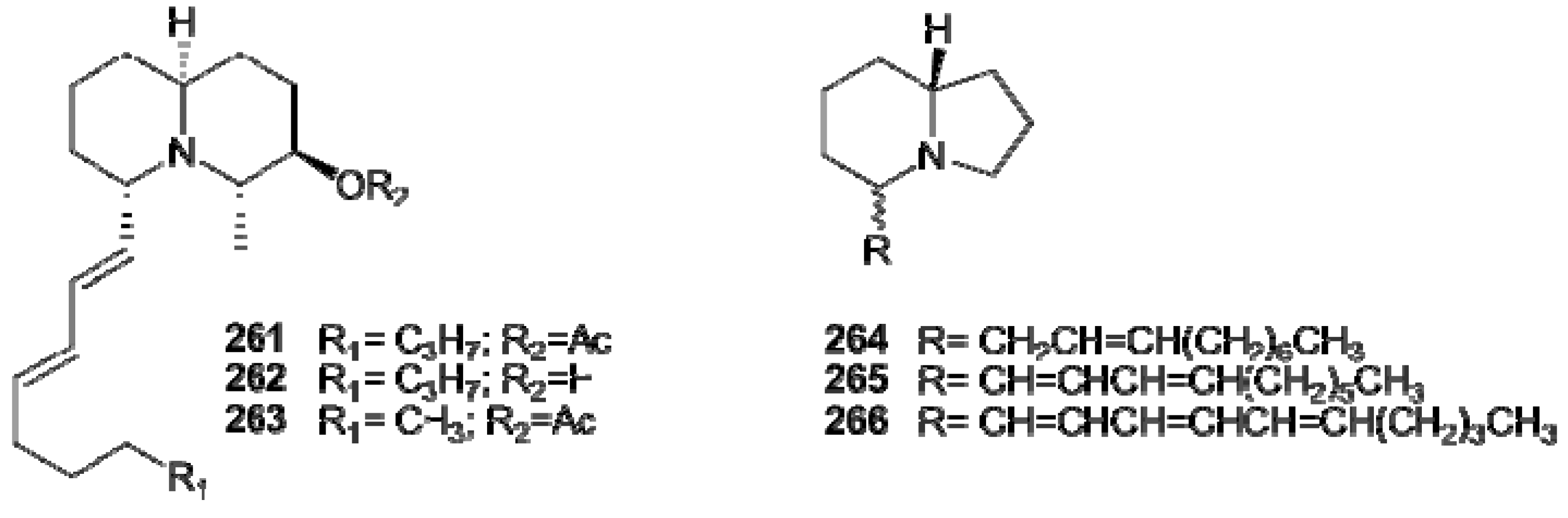

7. Protoalkaloids



8. Dimeric Steroidal Alkaloids

9. Conclusions
Acknowledgments
References and Notes
- Bruening, R.C.; Oh, E.M.; Furukawa, J.; Nakanishi, K.; Kuetin, K. Isolation of tunichrome B-1, a reducing blood pigment of the Sea Squirt, Ascidia nigra. J. Nat. Prod. 1986, 49, 193–204. [Google Scholar] [CrossRef]
- Davidson, B.S. Ascidians: Producers of amino acid derived metabolites. Chem. Rev. 1993, 93, 1771–1791. [Google Scholar] [CrossRef]
- Martoja, R.; Gouzerh, P.; Monniot, F. Cytochemical studies of vanadium, tunichromes and related substances in ascidians, possible biological significance. Oceanogr. Mar. Biol. Ann. Rev. 1994, 32, 531–556. [Google Scholar]
- Hamada, T.; Asanuma, M.; Ueki, T.; Hayashi, F.; Kobayashi, N.; Yokoyama, S.; Michibata, H.; Hirota, H. Solution structure of vanabin2, a vanadium(IV)-binding protein from the vanadium rich ascidian, Ascidia sydneiensis samea. J. Am. Chem. Soc. 2005, 127, 4216–4222. [Google Scholar]
- Faulkner, D.J. Marine natural products: Metabolites of marine invertebrates. Nat. Prod. Rep. 2002, 19, 1–48, and earlier reports in the series. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237, and earlier reports in the series. [Google Scholar] [CrossRef]
- Marshall, K.M.; Barrows, L.R. Biological activities of pyridoacridines. Nat. Prod. Rep. 2004, 21, 731–751. [Google Scholar] [CrossRef]
- Ding, Q.; Chichak, K.; Lown, J.W. Pyrroloquinoline and pyridoacridine alkaloids from marine sources. Curr. Med. Chem. 1999, 6, 1–27. [Google Scholar]
- Delfourne, E.; Bastide, J. Marine pyridoacridine alkaloids and synthetic analogues as antitumour agents. Med. Res. Rev. 2003, 23, 234–252. [Google Scholar] [CrossRef]
- Dias, N.; Vezin, H.; Lansiaux, A.; Bailly, C. Topoisomerase inhibitors of marine origin and their potential use as anticancer agents. Top. Curr. Chem. 2005, 253, 89–108. [Google Scholar]
- Kim, J.; Pordesimo, E.O.; Toth, S.I.; Schmitz, F.J. Pantherinine, a cytotoxic aromatic alkaloid, and 7-deazainosine from the ascidian Aplidium pantherinum. J. Nat. Prod. 1993, 56, 1813–1816. [Google Scholar] [CrossRef]
- Kobayashi, J.; Cheng, J.; Walchli, M.R.; Nakamura, H.; Hirata, Y.; Sasaki, T.; Ohizumi, Y. Cystodytins A, B, and C, novel tetracyclic aromatic alkaloids with potent antineoplastic activity from the Okinawan tunicate Cystodytes dellechiajei. J. Org. Chem. 1988, 53, 1800–1804. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tsuda, M.; Tanabe, A.; Ishibashi, M.; Cheng, J.F.; Yamamura, S.; Sasaki, T. Cystodytins D-I, new cytotoxic tetracyclic aromatic alkaloids from the Okinawan marine tunicate Cystodytes dellechiajei. J. Nat. Prod. 1991, 54, 1634–1638. [Google Scholar]
- McDonald, L.A.; Eldredge, G.S.; Barrows, L.R.; Ireland, C.M. Inhibition of Topoisomerase II catalytic activity by pyridoacridine alkaloids from a Cystodytes sp. ascidian: A mechanism for the apparent intercalator-induced inhibition of Topoisomerase II. J. Med. Chem. 1994, 37, 3819–3827. [Google Scholar] [CrossRef]
- Appleton, D.R.; Pearce, A.N.; Lambert, G.; Babcock, R.C.; Copp, B.R. Isodiplamine, cystodytin K and lissoclinidine: Novel bioactive alkaloids from the New Zealand ascidian Lissoclinum notti. Tetrahedron 2002, 58, 9779–9783. [Google Scholar] [CrossRef]
- Charyulu, G.A.; McKee, T.C.; Ireland, C.M. Diplamine, a cytotoxic polyaromatic alkaloid from the tunicate Diplosoma sp. Tetrahedron Lett. 1989, 30, 4201–4202. [Google Scholar]
- Clement, J.A.; Kitagaki, J.; Yang, Y.; Saucedo, C.J.; O'Keefe, B.R.; Weissman, A.M.; McKee, T.C.; McMahon, J.B. Discovery of new pyridoacridine alkaloids from Lissoclinum cf. badium that inhibit the ubiquitin ligase activity of Hdm2 and stabilize p53. Bioorg. Med. Chem. 2008, 16, 10022–10028. [Google Scholar]
- Searle, P.A.; Molinski, T.F. Five new alkaloids from the tropical ascidian, Lissoclinum sp. lissoclinotoxin A is chiral. J. Org. Chem. 1994, 59, 6600–6605. [Google Scholar] [CrossRef]
- Rudi, A.; Benayahu, Y.; Goldberg, I.; Kashman, Y. Alkaloid metabolites of the marine tunicate Eudistoma sp.: Segoline A, isosegoline A and norsegoline. Tetrahedron Lett. 1988, 29, 3861–3862. [Google Scholar]
- Rudi, A.; Kashman, Y. Six new alkaloids from the purple Red Sea tunicate Eudistoma sp. J. Org. Chem. 1989, 54, 5331–5337. [Google Scholar] [CrossRef]
- Molinski, T.F.; Ireland, C.M. Varamines A and B, new cytotoxic thioalkaloids from Lissoclinum vareau. J. Org. Chem. 1989, 54, 4256–4259. [Google Scholar] [CrossRef]
- Copp, B.R.; Jompa, J.; Tahir, A.; Ireland, C.M. Styelsamines A-D: New tetracyclic pyridoacridine alkaloids from the Indonesian ascidian Eusynstyela latericius. J. Org. Chem. 1998, 63, 8024–8026. [Google Scholar] [CrossRef]
- Einat, M.; Nagler, A.; Lishner, M.; Amiel, A.; Yarkoni, S.; Rudi, A.; Gellerman, G.; Kashman, Y.; Fabian, I. Potent antileukemic activity of the novel agents norsegoline and dibezine. Clin. Cancer Res. 1995, 1, 823–829. [Google Scholar]
- Kobayashi, J.; Cheng, J.F.; Nakamura, H.; Ohizumi, Y.; Hirata, Y.; Sasaki, T.; Ohta, T.; Nozoe, S. Ascididemin, a novel pentacyclic aromatic alkaloid with potent antileukemic activity from the Okinawan tunicate Didemnum sp. Tetrahedron Lett. 1988, 29, 1177–1180. [Google Scholar]
- Schmitz, F.J.; DeGuzman, F.S.; Hossain, M.B.; Van der Helm, D. Cytotoxic aromatic alkaloids from the ascidian Amphicarpa meridiana and Leptoclinides sp.: Meridine and 11-hydroxyascididemin. J. Org. Chem. 1991, 56, 804–808. [Google Scholar] [CrossRef]
- Cooray, N.M.; Scheuer, P.J.; Parkanyi, L.; Clardy, J. Shermilamine A: A pentacyclic alkaloid from a tunicate. J. Org. Chem. 1988, 53, 4619–4620. [Google Scholar] [CrossRef]
- Carroll, A.R.; Cooray, N.M.; Poiner, A.; Scheuer, P.J. A second shermilamine alkaloid from a tunicate Trididemnum sp. J. Org. Chem. 1989, 54, 4231–4232. [Google Scholar] [CrossRef]
- Koren-Goldshlager, G.; Aknin, M.; Gaydou, E.M.; Kashman, Y. Three new alkaloids from the marine tunicate Cystodytes violatinctus. J. Org. Chem. 1998, 63, 4601–4603. [Google Scholar] [CrossRef]
- López-Legentil, S.; Dieckmann, R.; Bontemps-Subielos, N.; Turon, X.; Banaigs, B. Qualitative analysis of alkaloids in color morphs of Cystodites (Ascidiacea). Biochem. Syst. Ecol. 2005, 33, 1107–1119. [Google Scholar] [CrossRef]
- Carroll, A.R.; Scheuer, P.J. Kuanoniamines A, B, C, and D: Pentacyclic alkaloids from a tunicate and its prosobranch mollusk predator Chelynotus semperi. J. Org. Chem. 1990, 55, 4426–4431. [Google Scholar]
- Nilar, P.J.S.; Carté, B.K.; Butler, M.S. Three new pyridoacridine typa alkaloids from a Singaporean ascidian. J. Nat. Prod. 2002, 65, 1198–1200. [Google Scholar] [CrossRef]
- Torres, Y.R.; Bugni, T.S.; Berlinck, R.G.S.; Ireland, C.M.; Magalhaes, A.; Ferreira, A.G.; Moreira da Rocha, R. Sebastianines A and B, novel biologically active pyridoacridine alkaloids from the Brazilian ascidian Cystodytes dellechiajei. J. Org. Chem. 2002, 67, 5429–5432. [Google Scholar] [CrossRef]
- Plubrukarn, A.; Davidson, B.S. Arnoamines A and B, new cytotoxic pentacyclic pyridoacridine alkaloids from the ascidian Cystodytes sp. J. Org. Chem. 1998, 63, 1657–1659. [Google Scholar] [CrossRef]
- Viracaoundin, I.; Faure, R.; Gaydou, E.M.; Aknin, M. A new alkaloid from the purple Indian Ocean tunicate Eudistoma bituminis. Tetrahedron Lett. 2001, 42, 2669–2671. [Google Scholar] [CrossRef]
- He, H.Y.; Faulkner, D.J. Eudistones A and B: Two novel octacyclic alkaloids from a seychelles tunicate Eudistoma sp. J. Org. Chem. 1991, 56, 5369–5371. [Google Scholar] [CrossRef]
- Rudi, A.; Benayahu, Y.; Goldberg, I.; Kashman, Y. Eilatin, a novel alkaloid from the marine tunicate Eudistoma sp. Tetrahedron Lett. 1988, 29, 6655–6656. [Google Scholar]
- Shochet, N.R.; Rudi, A.; Kashman, Y.; Hod, Y.; El-Maghrabi, M.R.; Spector, I. Novel marine alkaloids from the tunicate Eudistoma sp. are potent regulators of cellular growth and differentiation and affect cAMP-mediated processes. J. Cell. Physiol. 1993, 157, 481–492. [Google Scholar] [CrossRef]
- Luedtke, N.W.; Hwang, J.S.; Nava, E.; Gut, D.; Kol, M.; Tor, Y. The DNA and RNA specificity of eilatin Ru(II) complexes as compared to eilatin and ethidium bromide. Nucl. Acids Res. 2003, 31, 5732–5740. [Google Scholar] [CrossRef]
- Kobayashi, J.; Harbour, G.C.; Gilmore, J.; Rinehart, K.L., Jr. Eudistomins A, D, G, H, I, J, M, N, O, P, and Q, bromo, hydroxy, pyrrolyl and iminoazepino b-carbolines from the antiviral Caribbean tunicate Eudistoma olivaceum. J. Am. Chem. Soc. 1984, 106, 1526–1528. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Kobayashi, J.; Harbour, G.C.; Gilmore, J.; Mascal, M.; Holt, T.G.; Shield, L.S.; Lafargue, F. Eudistomins A-Q, β-carbolines from the antiviral Caribbean tunicate Eudistoma olivaceum. J. Am. Chem. Soc. 1987, 109, 3378–3387. [Google Scholar] [CrossRef]
- Kinzer, K.F.; Cardellina, J.H., II. Three new β-carbolines from the Bermudian tunicate Eudistoma olivaceum. Tetrahedron Lett. 1987, 28, 925–926. [Google Scholar]
- Schupp, P.; Poehner, T.; Edrada, R.; Ebel, R.; Berg, A.; Wray, V.; Proksch, P. Eudistomins W and X, two new β-carbolines from the Micronesian tunicate Eudistoma sp. J. Nat. Prod. 2003, 66, 272–275. [Google Scholar] [CrossRef]
- Wang, W.; Nam, S.; Lee, B.; Kang, H. β-Carboline alkaloids from a Korean tunicate Eudistoma sp. J. Nat. Prod. 2008, 71, 163–166. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Kobayashi, J.; Harbour, G.C.; Hughes, R.G., Jr.; Mizsak, S.A.; Scahill, T.A. Eudistomins C, E, K, and L, potent antiviral compounds containing a novel oxathiazepine ring from the Caribbean tunicate Eudistoma olivaceum. J. Am. Chem. Soc. 1984, 106, 1524–1526. [Google Scholar] [CrossRef]
- Kobayashi, J.; Nakamura, H.; Ohizumi, Y.; Hirata, Y. Eudistomidin-A, a novel calmodulin antagonist from the Okinawan tunicate Eudistoma glaucus. Tetrahedron Lett. 1986, 27, 1191–1194. [Google Scholar] [CrossRef]
- Kobayashi, J.; Cheng, J.F.; Oht, T.; Nozoe, S.; Ohizumi, Y.; Sasaki, T. Eudistomidins B, C, and D: Novel antileukemic alkaloids from the Okinawan marine tunicate Eudistoma glaucus. J. Org. Chem. 1990, 55, 3666–3670. [Google Scholar] [CrossRef]
- Murata, O.; Shigemori, H.; Ishibashi, M.; Sugama, K.; Hayashi, K.; Kobayashi, J. Eudistomidins E and F, new β-carboline alkaloids from the Okinawan marine tunicate Eudistoma glaucus. Tetrahedron Lett. 1991, 32, 3539–3542. [Google Scholar]
- Takahashi, Y.; Ishiyama, H.; Kubota, T.; Kobayashi, J. Eudistomidin G, a new b-carboline alkaloid from the Okinawan marine tunicate Eudistoma glaucus and structure revision of eudistomidin B. Bioorg. Med. Chem. Lett. 2010, 20, 4100–4103. [Google Scholar]
- Adesanya, S.A.; Chbani, M.; Pais, M.; Debitus, C. Brominated β-carbolines from the marine tunicate Eudistoma album. J. Nat. Prod. 1992, 55, 525–527. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. New cytotoxic N-methylated β-carboline alkaloids from the marine ascidian Eudistoma gilboverde. J. Nat. Prod. 2001, 4, 1454–1456. [Google Scholar]
- Debitus, C.; Laurent, D.; Pais, M. Alkaloids from an ascidian of New Caledonia, Eudistoma fragum. J. Nat. Prod. 1988, 51, 99–801. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Jompa, J.; Tahir, A.; Ireland, C.M. Trypargine alkaloids from a previously undescribed Eudistoma sp. ascidian. J. Nat. Prod. 1999, 62, 794–797. [Google Scholar] [CrossRef]
- Lake, R.J.; Brennan, M.M.; Blunt, J.W.; Munro, M.H.G.; Pannell, L.K. Eudistomin K sulfoxide. An antiviral sulfoxide from the New Zealand ascidian Ritterella sigillinoides. Tetrahedron Lett. 1988, 29, 2255–2256. [Google Scholar]
- Lake, R.J.; Blunt, J.W.; Munro, M.H.G. Eudistomins from the New Zealand ascidian Ritterella sigillinoides. Aust. J. Chem. 1989, 42, 1201–1206. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Quinn, R.J. Eudistomin V, a new β-Carboline from the Australian ascidian Pseudodistoma aureum. J. Nat. Prod. 1998, 61, 959–960. [Google Scholar] [CrossRef]
- Chbani, M.; Pais, M.; Delauneux, J.M.; Debitus, C. Brominated indole alkaloids from the marine tunicate Pseudodistoma arborescens. J. Nat. Prod. 1993, 56, 99–104. [Google Scholar]
- Rashid, M.A.; Gustafson, K.R.; Cartner, L.K.; Pannell, L.K.; Boyd, M.R. New nitrogenous constituents from the South African marine Ascidian Pseudodistoma sp. Tetrahedron 2001, 57, 5751–5755. [Google Scholar] [CrossRef]
- Schumacher, R.W.; Davidson, B.S. Didemnolines-D, new N9-substituted β-carbolines from the marine ascidian Didemnum sp. Tetrahedron 1995, 51, 10125–10130. [Google Scholar] [CrossRef]
- Oku, N.; Matsunaga, S.; Fusetani, N. Shishijimicins A-C, novel enediyne antitumor antibiotics from the ascidian Didemnum proliferum. J. Am. Chem. Soc. 2003, 125, 2044–2045. [Google Scholar] [CrossRef]
- Kearns, P.S.; Coll, J.C.; Rideout, J.A. A β-carboline dimer from an ascidian, Didemnum sp. J. Nat. Prod. 1995, 58, 1075–1076. [Google Scholar] [CrossRef]
- Kearns, P.S.; Rideout, J.A. Nonsymmetrical β-carboline dimers from an ascidian, Didemnum sp. J. Nat. Prod. 2008, 71, 1280–1282. [Google Scholar]
- Foderaro, T.A.; Barrows, L.R.; Lassota, P.; Ireland, C.M. Bengacarboline, a new β-carboline from a marine ascidian Didemnum sp. J. Org. Chem. 1997, 62, 6064–6065. [Google Scholar] [CrossRef]
- Ravinder, K.; Reddy, A.V.; Krishnaiah, P.; Ramesh, P.; Ramakrishna, S.; Laatsch, H.; Venkateswarlu, Y. Isolation and synthesis of a novel β-carboline guanidine derivative tiruchanduramine from the Indian ascidian Synoicum macroglossum. Tetrahedron Lett. 2005, 46, 5475–5478. [Google Scholar]
- Badre, A.; Boulanger, A.; Abou-Mansour, E.; Banaigs, B.; Combaut, G.; Francisco, C. Eudistomin U and isoeudistomin U, new alkaloids from the Caribbean asicidan Lissoclinum fragile. J. Nat. Prod. 1994, 57, 528–533. [Google Scholar]
- Shen, G.Q.; Baker, B.J. Biosynthetic studies of the eudistomins in the tunicate Eudistoma olivaceum. Tetrahedron Lett. 1994, 35, 1141–1144. [Google Scholar] [CrossRef]
- Che, C.T. Marine products as a source of antiviral drug leads. Drug Dev. Res. 1991, 23, 201–218. [Google Scholar] [CrossRef]
- Van Maarseveen, J.H.; Hermkens, P.H.; De Clercq, E.; Balzarini, J.; Scheeren, H.W.; Kruse, C.G. Antiviral and antitumor structure-activity relationship studies on tetracyclic eudistomines. J. Med. Chem. 1992, 35, 3223–3230. [Google Scholar] [CrossRef]
- Massiot, G.; Nazabadioko, S.; Bliard, C. Structural revision of isoeudistomin U by total synthesis. J. Nat. Prod. 1995, 58, 1636–1639. [Google Scholar] [CrossRef]
- Moquin, C.; Guyot, M. Grossularine, a novel indole derivative from the marine tunicate, Dendrodoa grossularia. Tetrahedron Lett. 1984, 25, 5047–5048. [Google Scholar] [CrossRef]
- Moquin-Pattey, C.; Guyot, M. Grossularine-1 and grossularine-2, cytotoxic α-carbolines from the tunicate Dendrodoa grossularia. Tetrahedron 1989, 45, 3445–50. [Google Scholar] [CrossRef]
- Abas, S.A.; Hossain, M.B.; van der Helm, D.; Schmitz, F.J.; Laney, M.; Cabuslay, R.; Schatzman, R.C. Alkaloids from the tunicate Polycarpa aurata from Chuuk Atoll. J. Org. Chem. 1996, 61, 2709–2712. [Google Scholar] [CrossRef]
- Niwa, H.; Yoshida, Y.; Yamada, K. A brominated quinazolinedione from the marine tunicate Pyura sacciformis. J. Nat. Prod. 1988, 51, 343–344. [Google Scholar] [CrossRef]
- Wratten, S.J.; Wolfe, M.S.; Andersen, R.J.; Faulkner, D.J. Antibiotic metabolites from a marine pseudomonad. Antimicrob. Agents Chemother. 1977, 11, 411–414. [Google Scholar]
- Kinnel, R.B.; Scheuer, P.J. 11-Hydroxystaurosporine: A highly cytotoxic, powerful protein kinase C inhibitor from a tunicate. J. Org. Chem. 1992, 57, 6327–6329. [Google Scholar]
- Horton, P.A.; Longley, R.E.; Mc Connell, O.J.; Ballas, L.M. Staurosporine aglycon (K252-c) and arcyriaflavin A from the marine ascidian, Eudistoma sp. Experientia 1994, 50, 843–845. [Google Scholar] [CrossRef]
- Schupp, P.; Eder, C.; Proksch, P.; Wray, V.; Schneider, B.; Herderich, M.; Paul, V. Staurosporine derivatives from the ascidian Eudistoma toealensis and its predatory flatworm Pseudoceros sp. J. Nat. Prod. 1999, 62, 959–962. [Google Scholar]
- Schupp, P.; Steube, K.; Meyer, C.; Proksch, P. Anti-proliferative effects of new staurosporine derivatives isolated from a marine ascidian and its predatory flatworm. Cancer Lett. 2001, 174, 165–172. [Google Scholar] [CrossRef]
- Schupp, P.; Proksch, P.; Wray, V. Further new staurosporine derivatives from the ascidian Eudistoma toealensis and its predatory flatworm Pseudoceros sp. J. Nat. Prod. 2002, 65, 295–298. [Google Scholar] [CrossRef]
- Reyes, F.; Fernandez, R.; Rodriguez, A.; Bueno, S.; de Eguilior, C.; Francesch, A.; Cuevas, C. Cytotoxic staurosporines from the marine ascidian Cystodytes solitus. J. Nat. Prod. 2008, 71, 1046–1048. [Google Scholar] [CrossRef]
- Omura, S.; Sasaki, Y.; Iwai, Y.; Takeshima, H. Staurosporine, a potentially important gift from a microorganism. J. Antibiot. 1995, 48, 535–548. [Google Scholar] [CrossRef]
- Fahy, E.; Potts, B.C.M.; Faulkner, D.J.; Smith, K. 6-Bromotryptamine derivatives from the Gulf of California tunicate Didemnum candidum. J. Nat. Prod. 1991, 54, 564–569. [Google Scholar] [CrossRef]
- Aiello, A.; Borrelli, F.; Capasso, R.; Fattorusso, E.; Luciano, P.; Menna, M. Conicamin, a novel histamine antagonist from the Mediterranean tunicate Aplidium conicum. Bioorg. Med. Chem. Lett. 2003, 13, 4481–4483. [Google Scholar] [CrossRef]
- Roll, D.M.; Ireland, C.M. Citorellamine, a new bromoindole derivative from Polycitorella mariae. Tetrahedron Lett. 1985, 26, 4303–4306. [Google Scholar] [CrossRef]
- Moriarty, R.M.; Roll, D.M.; Ku, Y.Y.; Nelson, C.; Ireland, C.M. A revised structure for the marine bromoindole derivative citorellamine. Tetrahedron Lett. 1987, 28, 749–752. [Google Scholar] [CrossRef]
- Lindquist, N.; Fenical, W. Polyandrocarpamides A-D, novel metabolites from the marine ascidian Polyandrocarpa sp. Tetrahedron Lett. 1990, 31, 2521–2524. [Google Scholar] [CrossRef]
- Heitz, S.; Durgeat, M.; Guyot, M.; Brassy, C.; Bachet, B. New indolic derivative of 1,2,4-thiadiazole, isolated from a tunicate (Dendrodoa grossularia). Tetrahedron Lett. 1980, 21, 1457–1458. [Google Scholar] [CrossRef]
- Guyot, M.; Meyer, M. An 3-indolyl-4H-imidazol-4-one from the tunicate Dendrodoa grossularia. Tetrahedron Lett. 1986, 27, 2621–2622. [Google Scholar] [CrossRef]
- Bergmann, T.; Schories, D.; Steffan, B. Alboinon, an oxadiazinone alkaloid from the ascidian Dendrodoa grossularia. Tetrahedron 1997, 53, 2055–2060. [Google Scholar] [CrossRef]
- Loukaci, A.; Guyot, M.; Chiaroni, A.; Riche, C. A new indole alkaloid from the marine tunicate Dendrodoa grossularia. J. Nat. Prod. 1998, 61, 519–522. [Google Scholar] [CrossRef]
- Franco, L.H.; Joffe, E.B.; Puricelli, L.; Tatian, M.; Seldes, A.M.; Palermo, J.A. Indole alkaloids from the tunicate Aplidium meridianum. J. Nat. Prod. 1998, 61, 1130–1132. [Google Scholar] [CrossRef]
- Seldes, A.M.; Brasco, M.F.R.; Franco, L.H.; Palermo, J.A. Identification of two meridianins from the crude extract of the tunicate Aplidium meridianum by tandem mass spectrometry. Nat. Prod. Res. 2007, 2, 555–563. [Google Scholar]
- Gompel, M.; Leost, M.; De Kier, J.E.B.; Puricelli, L.; Franco, L.H.; Palermo, J.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar] [CrossRef]
- Reyes, F.; Fernandez, R.; Rodriguez, A.; Francesch, A.; Taboada, S.; Avila, C.; Cuevas, C. Aplicyanins A-F, new cytotoxic bromoindole derivatives from the marine tunicate Aplidium cyaneum. Tetrahedron 2008, 64, 5119–5123. [Google Scholar] [CrossRef]
- Appleton, D.R.; Page, M.J.; Lambert, G.; Berridge, M.V.; Copp, B.R. Kottamides A-D: Novel bioactive imidazolone-containing alkaloids from the New Zealand ascidian Pycnoclavella kottae. J. Org. Chem. 2002, 67, 5402–5404. [Google Scholar]
- Appleton, D.R.; Copp, B.R. Kottamide E, the first example of a natural product bearing the amino acid 4-amino-1,2-dithiolane-4-carboxylic acid (Adt). Tetrahedron Lett. 2003, 44, 8963–8965. [Google Scholar] [CrossRef]
- Copp, B.R.; Ireland, C.M.; Barrows, L.R. Wakayin: A novel cytotoxic pyrroloiminoquinone alkaloid from the ascidian Clavelina species. J. Org. Chem. 1991, 56, 4596–4597. [Google Scholar] [CrossRef]
- Vervoort, H.C.; Richards-Gross, S.E.; Fenical, W.; Lee, A.Y.; Clardy, J. Didemnimides A-D: Novel predator-deterrent alkaloids from the Caribbean mangrove ascidian Didemnum conchyliatum. J. Org. Chem. 1997, 62, 1486–1490. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Britton, R.; Piers, E.; Lim, L.; Roberge, M.; Moreira da Rocha, R.; Andersen, R.J. Granulatimide and Isogranulatimide, aromatic alkaloids with G2 checkpoint inhibition activity isolated from the Brazilian Ascidian Didemnum granulatum: Structure elucidation and synthesis. J. Org. Chem. 1998, 63, 9850–9856. [Google Scholar]
- Vervoort, H.C.; Fenical, W.; Keifer, P.A. A cyclized didemnimide alkaloid from the Caribbean Ascidian Didemnum conchyliatum. J. Nat. Prod. 1999, 62, 389–391. [Google Scholar] [CrossRef]
- Britton, R.; de Oliveira, J.H.H.L.; Andersen, R.J.; Berlinck, R.G.S. Granulatimide and 6-bromogranulatimide, minor alkaloids of the Brazilian ascidian Didemnum granulatum. J. Nat. Prod. 2001, 64, 254–255. [Google Scholar] [CrossRef]
- Henon, H.; Messaoudi, S.; Anizon, F.; Aboab, B.; Kucharczyk, N.; Leonce, S.; Golsteyn, R.M.; Pfeiffer, B.; Prudhomme, M. Bis-imide granulatimide analogues as potent checkpoint 1 kinase inhibitors. Eur. J. Pharmacol. 2007, 554, 106–112. [Google Scholar]
- Sato, H.; Tsuda, M.; Watanabe, K.; Kobayashi, J. Rhopaladins A-D, new indole alkaloids, from marine tunicate Rhopalaea sp. Tetrahedron 1998, 54, 8687–8690. [Google Scholar] [CrossRef]
- Sasaki, T.; Ohtani, I.I.; Tanaka, J.; Higa, T. Iheyamines, new cytotoxic bisindole pigments from a colonial ascidian, Polycitorella sp. Tetrahedron Lett. 1999, 40, 303–306. [Google Scholar] [CrossRef]
- Segraves, N.L.; Lopez, S.; Johnson, T.A.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Structures and cytotoxicities of fascaplysin and related alkaloids from two marine phyla-Fascaplysinopsis sponges and Didemnum tunicates. Tetrahedron Lett. 2003, 4, 3471–3475. [Google Scholar]
- Segraves, N.L.; Robinson, S.J.; Garcia, D.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Comparison of fascaplysin and related alkaloids: A study of structures, cytotoxicities, and sources. J. Nat. Prod. 2004, 67, 783–792. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Baranova, O.V.; Balaneva, N.N.; Fedorov, S.N.; Radchenko, O.S.; Dubovitskii, S.V. The first syntheses of 3-bromofascaplysin, 10-bromofascaplysin and 3,10-dibromofascaplysin -marine alkaloids from Fascaplysinopsis reticulata and Didemnum sp. by application of a simple and effective approach to the pyrido[1,2-a:3,4-b']diindole system. Tetrahedron Lett. 2007, 48, 7998–8000. [Google Scholar] [CrossRef]
- Ireland, C.M.; Durso, A.R., Jr. N,N1-Diphenethilurea, a metabolite from the marine ascidian Didemnum ternatanu. J. Nat. Prod. 1981, 44, 360–361. [Google Scholar] [CrossRef]
- Ford, P.W.; Davidson, B.S. Plakinidine D, a new pyrroloacridine alkaloid from the ascidian Didemnum rubeum. J. Nat. Prod. 1997, 60, 1051–1053. [Google Scholar] [CrossRef]
- Smith, C.J.; Venables, D.A.; Hopmann, C.; Salomon, C.E.; Jompa, J.; Tahir, A.; Faulkner, D.J.; Ireland, C.M. Plakinidine D, a new pyrroloacridine Alkoaloid from two ascidians of the genus Didemnum. J. Nat. Prod. 1997, 60, 1048–1050. [Google Scholar] [CrossRef]
- Lindsay, B.S.; Battershill, C.N.; Copp, B.R. Isolation of 2-(31-bromo-41-hydroxyphenol)ethanamine from the New Zealand ascidian Cnemidocarpa bicornuta. J. Nat. Prod. 1998, 61, 857–858. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Imperatore, C.; Menna, M.; Müller, W.E.G. Iodocionin, a cytotoxic iodinated metabolite from the mediterranean ascidian Ciona edwardsii. Mar. Drugs 2010, 8, 285–291. [Google Scholar] [CrossRef]
- Solano, G.; Motti, C.A.; Jaspars, M. New iodotyramine derivatives from Didemnum rubeum. Tetrahedron 2009, 65, 7482–7486. [Google Scholar]
- Pearce, A.N.; Chia, E.W.; Berridge, M.V.; Maas, E.W.; Page, M.J.; Harper, J.L.; Webb, V.L.; Copp, B.R. Orthidines A-E, tubastrine, 3,4-dimethoxyphenethyl-β-guanidine, and 1,14-sperminedihomovanillamide: Potential anti-inflammatory alkaloids isolated from the New Zealand ascidian Aplidium orthium that act as inhibitors of neutrophil respiratory burst. Tetrahedron 2008, 64, 5748–5755. [Google Scholar]
- Wessels, M.; König, G.M.; Wright, A.D. New 4-methoxybenzoyl derivatives from the ascidian Polycarpa aurata. J. Nat. Prod. 2001, 64, 1556–1558. [Google Scholar] [CrossRef]
- McDonald, L.A.; Swersey, J.C.; Ireland, C.M.; Carroll, A.R.; Coll, J.C.; Bowden, B.F.; Fairchild, C.R.; Cornell, L. Botryllamides A-D, new brominated tyrosine derivatives from styelid ascidians of the genus Botryllus. Tetrahedron 1995, 51, 5237–5244. [Google Scholar]
- Rao, M.R.; Faulkner, D.J. Botryllamides E-H, four new Tyrosine derivatives from the ascidian Botrylloides tyreum. J. Nat. Prod. 2004, 67, 1064–1066. [Google Scholar] [CrossRef]
- Henrich, C.J.; Robey, R.W.; Takada, K.; Bokesch, H.R.; Bates, S.E.; Shukla, S.; Ambudkar, S.V.; McMahon, J.B.; Gustafson, K.R. Botryllamides: Natural product inhibitors of ABCG2. Chem. Biol. 2009, 4, 637–647. [Google Scholar]
- Yin, S.; Cullinane, C.; Carroll, A.R.; Quinn, R.J.; Davis, R.A. Botryllamides K and L, new tyrosine derivatives from the Australian ascidian Aplidium altarium. Tetrahedron Lett. 2010, 51, 3403–3405. [Google Scholar]
- Takada, K.; Inamura, N.; Gustafson, K.R.; Henrich, C.J. Synthesis and structure-activity relationship of botryllamides that block the ABCG2 multidrug transporter. Bioorg. Med. Chem. Lett. 2010, 20, 330–1333. [Google Scholar] [CrossRef]
- Arabshahi, L.; Schmitz, F.J. Thiazole and imidazole metabolites from the ascidian Aplydium pliciferum. Tetrahedron Lett. 1988, 29, 1099–1102. [Google Scholar] [CrossRef]
- Davis, R.A.; Aalbersberg, W.; Meo, S.; Moreira da Rocha, R.; Ireland, C.M. The isolation and synthesis of polyandrocarpamines A and B. Two new 2-aminoimidazolone compounds from the Fijian ascidian, Polyandrocarpa sp. Thetrahedron 2002, 58, 3263–3269. [Google Scholar] [CrossRef]
- Hirsch, S.; Miroz, A.; McCarthy, P.; Kashman, Y. Etzionin, a new antifungal metabolite from a red sea tunicate. Tetrahedron Lett. 1989, 30, 4291–4294. [Google Scholar] [CrossRef]
- Kobayashi, J.; Cheng, J.; Kikuchi, Y.; Ishibashi, M.; Yamamura, S.; Ohizumi, Y.; Ohta, T.; Nozoe, S. Rigidin, a novel alkaloid with calmodulin antagonistic activity from the okinawan marine tunicate Eudistoma rigida. Tetrahedron Lett. 1990, 31, 4617–4620. [Google Scholar] [CrossRef]
- Tsuda, M.; Nozawa, K.; Shimbo, K.; Kobayashi, J. Rigidins B-D, new pyrrolopyrimidine alkaloids from a tunicate Cystodytes species. J. Nat. Prod. 2003, 66, 292–294. [Google Scholar]
- Davis, R.A.; Christensen, L.V.; Richardson, A.D.; Moreira da Rocha, R.; Ireland, C.M. Rigidin E, a new pyrrolopyrimidine alkaloids from a Papua New Guinea tunicate Eudistoma species. Mar. Drugs 2003, 1, 27–33. [Google Scholar] [CrossRef]
- Durán, R.; Zubía, E.; Ortega, M.G.; Naranjo, S.; Salvá, J. Novel alkaloids from the red ascidian Botryllus leachi. Tetrahedron 1999, 55, 13225–13232. [Google Scholar] [CrossRef]
- Garrido, L.; Zubía, E.; Ortega, M.G.; Salvá, J. Haouamines A and B: A new class of alkaloids from the ascidian Aplidium haouarianum. J. Org. Chem. 2003, 68, 293–299. [Google Scholar]
- Andersen, R.J.; Faulkner, D.J.; He, C.H.; Van Duyne, G.D.; Clardy, J. Metabolites of the marine prosobranch mollusk Lamellaria sp. J. Am. Chem. Soc. 1985, 107, 5492–5495. [Google Scholar] [CrossRef]
- Lindquist, N.; Fenical, W.; Van Duyne, G.D.; Clardy, J. New alkaloids of the Lamellarin class from the marine ascidian Didemnum chartaceum. J. Org. Chem. 1988, 53, 4570–4574. [Google Scholar]
- Carroll, A.R.; Bowden, B.F.; Coll, J.C. Studies of Australian ascidians. I. Six new lamellarin-class alkaloids from a colonial ascidian, Didemnum sp. Aust. J. Chem. 1993, 46, 489–501. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Pierens, G.K.; Quinn, R.J. New lamellarin alkaloids from the Australian ascidian Didemnum chartaceum. J. Nat. Prod. 1999, 62, 419–424. [Google Scholar] [CrossRef]
- Reddy, M.V.R.; Rao, M.R.; Rhodes, D.; Hansen, M.S.T.; Rubins, K.; Bushman, F.D.; Venkateswarlu, Y.; Faulkner, D.J. Lamellarin α-20 sulfate, an inhibitor of HIV-1 integrase active against HIV-1 virus in cell culture. J. Med. Chem. 1999, 42, 1901–1907. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Reddy, V.L.N.; Venkataramana, G.; Ravinder, K.; Srinivasulu, M.; Raju, T.V.; Ravikumar, K.; Chandrasekar, D.; Ramakrishna, S.; Venkateswarlu, Y. New lamellarin alkaloids from the Indian ascidian Didemnum obscurum and their antioxidant properties. J. Nat. Prod. 2004, 67, 1168–1171. [Google Scholar]
- Reddy, S.M.; Srinivasulu, M.; Satyanarayana, N.; Kondapi, A.K.; Venkateswarlu, Y. New potent cytotoxic lamellarin alkaloids from Indian ascidian Didemnum obscurum. Tetrahedron 2005, 61, 9242–9247. [Google Scholar]
- Urban, S.; Butler, M.S.; Capon, R.J. Lamellarins O and P: New aromatic metabolites from the Australian marine sponge Dendrilla cactos. Aust. J. Chem. 1994, 47, 1919–1924. [Google Scholar] [CrossRef]
- Urban, S.; Hobbs, L.; Hooper, J.N.A.; Capon, R.J. Lamellarins Q and R: New aromatic metabolites from an Australian marine Sponge Dendrilla cactos. Aust. J. Chem. 1995, 48, 1491–1494. [Google Scholar] [CrossRef]
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.F. Lamellarins and related pyrrole-derived alkaloids from marine organisms. Chem. Rev. 2008, 108, 264–287. [Google Scholar] [CrossRef]
- Bailly, C. Lamellarins, from A to Z: A family of anticancer marine pyrrole alkaloids. Curr. Med. Chem. Anti-Cancer Agents 2004, 4, 363–378. [Google Scholar] [CrossRef]
- Kluza, J.; Marchetti, P.; Bailly, C. Lamellarin alkaloids: Structure and pharmacological properties. In Modern Alkaloids; Fattorusso, E., Taglialatela-Scafati, O., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 171–187. [Google Scholar]
- Tardy, C.; Facompre, M.; Laine, W.; Baldeyrou, B.; Garcia-Gravalos, D.; Francesch, A.; Mateo, C.; Pastor, A.; Jimenez, J.A.; Manzanares, I.; et al. Topoisomerase I-mediated DNA cleavage as a guide to the development of antitumor agents derived from the marine alkaloid lamellarin D: Triester derivatives incorporating amino acid residues. Bioorg. Med. Chem. 2004, 12, 1697–1712. [Google Scholar] [CrossRef]
- Marco, E.; Laine, W.; Tardy, C.; Lansiaux, A.; Iwao, M.; Ishibashi, F.; Bailly, C.; Gago, F. Molecular determinants of topoisomerase I poisoning by lamellarins: Comparison with camptothecin and structure-activity relationships. J. Med. Chem. 2005, 48, 3796–3807. [Google Scholar] [CrossRef]
- Facompre, M.; Tardy, C.; Bal-Mahieu, C.; Colson, P.; Perez, C.; Manzanares, I.; Cuevas, C.; Bailly, C. Lamellarin D: A novel potent inhibitor of topoisomerase I. Cancer Res. 2003, 63, 7392–7399. [Google Scholar]
- Marco, E.; Laine, W.; Tardy, C.; Lansiaux, A.; Iwao, M.; Ishibashi, F.; Bailly, C.; Gago, F. Molecular determinants of topoisomerase I poisoning by lamellarins: Comparison with camptothecin and structure-activity relationships. J. Med. Chem. 2005, 48, 3796–3807. [Google Scholar]
- Quesada, A.R.; Gravalos, M.D.G.; Puentes, J.L.F. Polyaromatic alkaloids from marine invertebrates as cytotoxic compounds and inhibitors of multidrug resistance caused by P-glycoprotein. Br. J. Cancer 1996, 74, 677–682. [Google Scholar] [CrossRef]
- Yoshida, W.Y.; Lee, K.K.; Carroll, A.R.; Scheuer, P.J. A complex pyrrolo-oxazinone and its iodo derivative isolated from a tunicate. Helv. Chim. Acta 1992, 75, 1721–1725. [Google Scholar] [CrossRef]
- Rudi, A.; Goldberg, I.; Stein, Z.; Frolow, F.; Benayahu, Y.; Schleyer, M.; Kashman, Y. Polycitone A and Polycitrins A and B: New alkaloids from the marine Ascidian Polycitor sp. J. Org. Chem. 1994, 59, 999–1003. [Google Scholar] [CrossRef]
- Rudi, A.; Evan, T.; Aknin, M.; Kashman, Y. Polycitone B and Prepolycitrin A: Two novel alkaloids from the marine ascidian Polycitor africanus. J. Nat. Prod. 2000, 63, 832–833. [Google Scholar]
- Kang, H.; Fenical, W. Ningalins A-D: Novel aromatic alkaloids from a western Australians ascidian of the genus Didemnum. J. Org. Chem. 1997, 62, 3254–3262. [Google Scholar] [CrossRef]
- Cuevas, C.; Francesch, A. Development of Yondelis® (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep. 2009, 26, 322–337. [Google Scholar] [CrossRef]
- Menchaca, R.; Martínez, V.; Rodríguez, A.; Rodríguez, N.; Flores, M.; Gallego, P.; Manzanares, I.; Cuevas, C. Synthesis of natural ecteinascidins (ET-729, ET-745, ET-759B, ET-736, ET-637, ET-594) from cyanosafracin B. J. Org. Chem. 2003, 68, 8859–8866. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Holt, T.G.; Fregeau, N.L.; Stroh, J.G.; Keifer, P.A.; Sun, F.; Li, L.H.; Martin, D.G. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: Potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4512–4515. [Google Scholar]
- Wright, A.E.; Forleo, D.A.; Gunawardana, G.P.; Gunasekera, S.P.; Koehn, F.E.; McConnell, O.J. Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4508–4512. [Google Scholar] [CrossRef]
- Guan, Y.; Sakai, R.; Rinehart, K.L.; Wang, A.H.J. Molecular and crystal structures of ecteinascidins: potent antitumor compounds from the Caribbean tunicate Ecteinascidia turbinata. J. Biomol. Struct. Dyn. 1993, 10, 793–818. [Google Scholar]
- Sakai, R.; Rinehart, K.L.; Guan, Y.; Wang, A.H.J. Additional antitumor ecteinascidins from a Caribbean tunicate: Crystal structures and activities in vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 11456–11460. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Sakai, R. Isolation, structure elucidation, and bioactivities of novel ecteinascidins from Ecteinascidia turbinata. US Pat. Appl. Publ. 005 2004. [Google Scholar]
- Suwanborirux, K.; Charupant, K.; Amnuoypol, S.; Pummangura, S.; Kubo, A.; Saito, N. Ecteinascidins 770 and 786 from the Thai Tunicate Ecteinascidia thurstoni. J. Nat. Prod. 2002, 65, 935–937. [Google Scholar]
- Sakai, R.; Jares-Erijman, E.A.; Manzanares, I.; Elipe, M.V.S.; Rinehart, K.L. Ecteinascidins: Putative biosynthetic precursors and absolute stereochemistry. J. Am. Chem. Soc. 1996, 118, 9017–9023. [Google Scholar]
- Aune, G.J.; Furuta, T.; Pommier, Y. Ecteinascidin 743: A novel anticancer drug with a unique mechanism of action. Anticancer Drugs 2002, 13, 545–555. [Google Scholar] [CrossRef]
- D’Incalci, M.; Erba, E.; Damia, G.; Galliera, E.; Carrassa, L.; Marchini, S.; Mantovani, R.; Tognon, G.; Fruscio, R.; Jimeno, J.; Faircloth, G.T. Unique features of the mode of action of ET-743. Oncologist 2002, 7, 210–216. [Google Scholar] [CrossRef]
- Fayette, J.; Coquard, I.R.; Alberti, L.; Boyle, H.; Meeus, P.; Decouvelaere, A.V.; Thiesse, P.; Sunyach, M.P.; Ranchere, D.; Blay, J.Y. ET-743: A novel agent with activity in soft-tissue sarcomas. Curr. Opin. Oncol. 2006, 18, 347–353. [Google Scholar] [CrossRef]
- Gajdos, C.; Elias, A. Trabectedin: Safety and efficacy in the treatment of advanced sarcoma. Clin. Med. Ins. Oncol. 2011, 5, 35–43. [Google Scholar] [CrossRef]
- Valoti, G.; Nicoletti, M.I.; Pellegrino, A.; Jimeno, J.; Hendriks, H.; D’Incalci, M.; Faircloth, G.; Giavazzi, R. Ecteinascidin-743, a new marine natural product with potent antitumor activity on human ovarian carcinoma xenografts. Clin. Cancer Res. 1998, 4, 1977–1983. [Google Scholar]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Ishibashi, M.; Ohizumi, Y.; Sasaki, T.; Nakamura, H.; Hirata, Y.; Kobayashi, J. Pseudodistomins A and B, novel antineoplastic piperidine alkaloids with calmodulin antagonistic activity from the Okinawan tunicate Pseudodistoma kanoko. J. Org. Chem. 1987, 52, 450–453. [Google Scholar] [CrossRef]
- Kiguchi, T.; Yuumoto, Y.; Ninomiya, I.; Naito, T.; Deki, K.; Ishibashi, M.; Kobayashi, J. Pseudodistomin B: Revised structure and first total synthesis. Tetrahedron Lett. 1992, 33, 7389–7390. [Google Scholar] [CrossRef]
- Ishibashi, M.; Deki, K.; Kobayashi, J. Revised structure of Pseudodistomin A, a piperidine alkaloid isolated from the Okinawan Tunicate Pseudodistoma kanoko. J. Nat. Prod. 1995, 58, 804–806. [Google Scholar] [CrossRef]
- Knapp, S.; Hale, J. Synthesis of (+)-tetrahydropseudodistomin. J. Org. Chem. 1993, 58, 2650–2651. [Google Scholar] [CrossRef]
- Naito, T.; Yuumoto, Y.; Ninomiya, I.; Kiguchi, T. First total synthesis of pseudodistomin tetrahydroacetate. Tetrahedron Lett. 1992, 33, 4033–4036. [Google Scholar] [CrossRef]
- Kobayashi, J.; Naitoh, K.; Doi, Y.; Deki, K.; Ishibashi, M. Pseudodistomin C, a new piperidine alkaloid with unusual absolute configuration from the Okinawan tunicate Pseudodistoma kanoko. J. Org. Chem. 1995, 60, 6941–6945. [Google Scholar] [CrossRef]
- Freyer, A.J.; Patil, A.D.; Killmer, L.; Troupe, N.; Mentzer, M.; Carte, B.; Faucette, L.; Johnson, L.K. Three new pseudodistomins, piperidine alkaloids from the ascidian Pseudodisto ma megalarva. J. Nat. Prod. 1997, 60, 986–990. [Google Scholar]
- Mc Coy, M.C.; Faulkner, D.J. Uoamines A and B, piperidine alkaloids from the ascidian Aplidium uouo. J. Nat. Prod. 2001, 64, 1087–1089. [Google Scholar] [CrossRef]
- Raub, M.F.; Cardellina, J.H., II.; Choudhary, M.I.; Ni, C.Z.; Clardy, J.; Alley, M.C. Clavepictines A and B: Cytotoxic quinolizidines from the tunicate Clavelina picta. J. Am. Chem. Soc. 1991, 113, 3178–3180. [Google Scholar] [CrossRef]
- Kong, F.; Faulkner, D.J. Pictamine, a quinolizidine alkaloid from the tunicate Clavelina picta. Tetrahedron Lett. 1991, 32, 3667–3668. [Google Scholar] [CrossRef]
- Raub, M.F.; Cardellina, J.H., II. The piclavines, antimicrobial indolizidines from the tunicate Clavelina picta. Tetrahedron Lett. 1992, 33, 2257–2260. [Google Scholar] [CrossRef]
- Blackman, A.J.; Li, C.P.; Hockless, D.C.R.; Skelton, B.W.; White, A.H. Cylindricines A and B, novel alkaloids from the ascidian Clavelina cylindrica. Tetrahedron 1993, 49, 8645–8656. [Google Scholar] [CrossRef]
- Li, C.P.; Blackman, A.J. Cylindricines C-G, perhydropyrrolo[2,1-j]quinolin-7-one alkaloids from the ascidian Clavelina cylindrica. Aust. J. Chem. 1994, 47, 1355–1361. [Google Scholar] [CrossRef]
- Li, C.P.; Blackman, A.J. Cylindricines H-K, novel alkaloids from the ascidian Clavelina cylindrica. Aust. J. Chem. 1995, 48, 955–965. [Google Scholar] [CrossRef]
- Issa, H.H.; Tanaka, J.; Rachmat, R.; Setiawan, A.; Trianto, A.; Higa, T. Polycitorols A and B, new tricyclic alkaloids from an ascidian. Mar. Drugs 2005, 3, 78–83. [Google Scholar]
- Biard, J.F.; Guyot, S.; Roussakis, C.; Verbist, J.F.; Vercauteren, J.; Weber, J.F.; Boukef, K. Lepadiformine, a new marine cytotoxic alkaloid from Clavelina lepadiformis. Tetrahedron Lett. 1994, 4, 2691–2694. [Google Scholar]
- Sauviat, M.P.; Vercauteren, J.; Grimaud, N.; Jugé, M.; Nabil, M.; Petit, J.Y.; Biard, J.F. Sensitivity of cardiac background inward rectifying K+ outward current (IK1) to the alkaloids lepadiformines A, B, and C. J. Nat. Prod. 2006, 69, 558–62. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Reichwein, R.; Carte, B.; Killmer, L.B.; Faucette, L.; Johnson, L.K.; Faulkner, D.J. Fasicularin, a novel tricyclic alkaloid from the ascidian Nephteis fasicularis with selective activity against a DNA repair-deficient organism. Tetrahedron Lett. 1997, 38, 363–364. [Google Scholar]
- Abe, H.; Aoyagi, S.; Kibayashi, C. First total synthesis of the marine alkaloid (±)-fascicularin and (±)-lepadiformine based on stereocontrolled intramolecular Acylnitroso-Diels-Alder-Reaction. J. Am. Chem. Soc. 2000, 122, 4583–4592. [Google Scholar] [CrossRef]
- Dutta, S.; Abe, H.; Aoyagi, S.; Kibayashi, C.; Gates, K.S. DNA damage by fasicularin. J. Am. Chem. Soc. 2005, 127, 15004–15005. [Google Scholar] [CrossRef]
- Kossuga, M.H.; MacMillan, J.B.; Rogers, E.W.; Molinski, T.F.; Nascimento, G.G.; Rocha, R.M.; Berlinck, R.G. (2S,3R)-2-aminododecan-3-ol, a new antifungal agent from the ascidian Clavelina oblonga. J. Nat. Prod. 2004, 67, 1879–1881. [Google Scholar]
- Aiello, A.; Fattorusso, E.; Giordano, A.; Menna, M.; Navarrete, C.; Muñoz, E. Clavaminols A-F, novel cytotoxic 2-amino-3-alkanols from the ascidian Clavelina phlegraea. Bioorg. Med. Chem. 2007, 15, 2920–2926. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Giordano, A.; Menna, M.; Navarrete, C.; Muñoz, E. Clavaminols G-N, six new marine sphingoids from the Mediterranean ascidian Clavelina phlegraea. Tetrahedron 2009, 65, 4384–4388. [Google Scholar] [CrossRef]
- Searle, P.A.; Molinski, T.F. Structure and absolute configuration of (R)-(E)-1-amino-tridec-5-en-2-ol, an antifungal amino alcohol from the ascidian Didemnum sp. J. Org. Chem. 1993, 58, 7578–7580. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Bapat, C.P.; Lithgow-Bertelloni, A.; Rinehart, K.L.; Sakai, R. Crucigasterins, new polyunsaturated amino alcohols from the mediterranean tunicate Pseudodistoma crucigaster. J. Org. Chem. 1993, 58, 5732–5737. [Google Scholar] [CrossRef]
- Garrido, L.; Zubía, E.; Ortega, M.J.; Naranjo, S.; Salvá, J. Obscuraminols, new unsaturated amino alcohols from the tunicate Pseudodistoma obscurum: structure and absolute configuration. Tetrahedron 2001, 57, 4579–4588. [Google Scholar] [CrossRef]
- Sata, N.U.; Fusetani, N. Amaminols A and B, new bicyclic amino alcohols from an unidentified tunicate of the family Polyclinidae. Tetrahedron Lett. 2000, 41, 489–492. [Google Scholar] [CrossRef]
- Kumpulainen, E.T.T.; Koskinen, A.M.P.; Rissanen, K. Total synthesis of Amaminol A: Establishment of the absolute stereochemistry. Org. Lett. 2007, 9, 5043–5045. [Google Scholar]
- Carter, G.; Rinehart, K.L. Aplidiasphingosine, an antimicrobial and antitumor terpenoid from an Aplidium sp. (marine tunicate). J. Am. Chem. Soc. 1978, 100, 7441–7442. [Google Scholar]
- Loukaci, A.; Bultel-Poncé, V.; Longeon, A.; Guyot, M. New Lipids from the Tunicate Cystodytes cf. dellechiajei, as PLA2 Inhibitors. J. Nat. Prod. 2000, 63, 799–802. [Google Scholar] [CrossRef]
- González, N.; Rodríguez, J.; Jiménez, C. Didemniserinolipids A-C, Unprecedented Serinolipids from the Tunicate Didemnum sp. J. Org. Chem. 1999, 64, 5705–5707. [Google Scholar] [CrossRef]
- Kiyota, H.; Dixon, D.J.; Luscombe, C.K.; Hettstedt, S.; Ley, S.V. Synthesis, structure revision, and absolute configuration of (+)-Didemniserinolipid B, a serinol marine natural product from a Tunicate Didemnum sp. Org. Lett. 2002, 4, 3223–3226. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ohashi, J.; Fujita, T.; Iwashita, T.; Nakao, Y.; Matsunaga, S.; Fusetani, N. Complete structure elucidation of shishididemniols, complex lipids with tyramine-derived tether and two serinol units, from a marine tunicate of the family didemnidae. J. Org. Chem. 2007, 72, 1218–1225. [Google Scholar]
- Kobayashi, H.; Miyata, Y.; Okada, K.; Fujita, T.; Iwashita, T.; Nakao, Y.; Fusetani, N.; Matsunaga, S. The structures of three new shishididemniols from a tunicate of the family Didemnidae. Tetrahedron 2007, 63, 6748–6754. [Google Scholar]
- Lievens, S.C.; Molinski, T.F. Sagittamides A and B, polyacetoxy long-chain acyl amino acids from a didemnid ascidian. Org. Lett. 2005, 7, 281–2284. [Google Scholar]
- Seike, H.; Ghosh, I.; Kishi, Y. Stereochemistry of Sagittamide A: Prediction and confirmation. Org. Lett. 2006, 8, 3865–3868. [Google Scholar]
- Fukuzawa, S.; Matsunaga, S.; Fusetani, N. Ritterazine A, a highly cytotoxic dimeric steroidal alkaloid, from the tunicate Ritterella tokioka. J. Org. Chem. 1994, 59, 6164–6166. [Google Scholar] [CrossRef]
- Fukuzawa, S.; Matsunaga, S.; Fusetani, N. Isolation and structure elucidation of ritterazines B and C, highly cytotoxic dimeric steroidal alkaloids, from the tunicate Ritterella tokioka. J. Org. Chem. 1995, 60, 608–614. [Google Scholar]
- Fukuzawa, S.; Matsunaga, S.; Fusetani, N. Bioactive marine metabolites. 71. Ten more ritterazines, cytotoxic steroidal alkaloids from the tunicate Ritterella tokioka. Tetrahedron 1995, 51, 6707–6716. [Google Scholar]
- Fukuzawa, S.; Matsunaga, S.; Fusetani, N. Isolation of 13 new ritterazines from the tunicate Ritterella tokioka and chemical transformation of ritterazine B. J. Org. Chem. 1997, 62, 4484–4491. [Google Scholar]
- Lee, S.; LaCour, T.G.; Lantrip, D.; Fuchs, P.L. Redox refunctionalization of steroid spiroketals. Structure correction of Ritterazine M. Org. Lett. 2002, 4, 31–316. [Google Scholar] [CrossRef]
- Pettit, G.R.; Inoue, M.; Kamano, Y.; Herald, D.L.; Arm, C.; Dufresne, C.; Christie, N.D.; Schmidt, J.M.; Doubek, D.L.; Krupa, T.S. Antineoplastic agents. 147. Isolation and structure of the powerful cell growth inhibitor cephalostatin 1. J. Am. Chem. Soc. 1988, 110, 2006–2007. [Google Scholar]
- Moser, B.R. Review of cytotoxic cephalostatins and ritterazines: Isolation and synthesis. J. Nat. Prod. 2008, 71, 487–491. [Google Scholar] [CrossRef]
- Lee, S.; LaCour, T.G.; Fuchs, P.L. Chemistry of trisdecacyclic pyrazine antineoplastics: The cephalostatins and ritterazines. Chem. Rev. 2009, 109, 2275–2314. [Google Scholar] [CrossRef]
- Komiya, T.; Fusetani, N.; Matsunaga, S.; Kubo, A.; Kaye, F.J.; Kelley, M.J.; Tamura, K.; Yoshida, M.; Fukuoka, M.; Nakagawa, K. Ritterazine B, a new cytotoxic natural compound, induces apoptosis in cancer cells. Cancer Chemother. Pharmacol. 2003, 51, 202–208. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Menna, M.; Fattorusso, E.; Imperatore, C. Alkaloids from Marine Ascidians. Molecules 2011, 16, 8694-8732. https://doi.org/10.3390/molecules16108694
Menna M, Fattorusso E, Imperatore C. Alkaloids from Marine Ascidians. Molecules. 2011; 16(10):8694-8732. https://doi.org/10.3390/molecules16108694
Chicago/Turabian StyleMenna, Marialuisa, Ernesto Fattorusso, and Concetta Imperatore. 2011. "Alkaloids from Marine Ascidians" Molecules 16, no. 10: 8694-8732. https://doi.org/10.3390/molecules16108694




