Abstract
Two samples of Cremanthodium stenactinium (Asteraceae) were collected in Sichuan Province, China. From the ethyl acetate extracts of the roots, three new eremophilane-type sesquiterpenoids and one new trinoreremophilane compound were isolated, together with other known eremophilanes. Their structures were determined based on the spectroscopic data. This is the first report of isolation of eremophilane-type compounds from the genus Cremanthodium.
1. Introduction
Plant species belonging to the genus Cremanthodium (Asteraceae) are known to grow in high mountain areas and to be very small in size [,]. To date there are few reports about these species, presumably due to the difficulty of collection and the scarcity of suitably sized samples. Bisabolane- and oplopane-type sesquiterpenoids and aromatic compounds were isolated from Cremanthodium ellisii [,,,,], bisabolane-type and steroids from C. discoideum [,], triterpenoids from C. potaninii [], and some hydrocarbons from C. pleurocaule []. However, there are no reports about the phytochemicals of C. stenactinium. We have been investigating both inter- and intra-specific diversity of Ligularia [,,,,,,]. In 2009, we had an opportunity to collect two samples of C. stenactinium at different locations in Sichuan Province of China. From the EtOAc extracts of the root we have now isolated four new compounds, three eremophilanes 1–3 and trinoreremophilane 4 (Figure 1), and their structures have been determined based on the spectroscopic data.
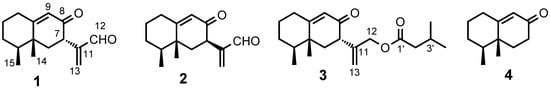
Figure 1.
New compounds isolated in this work.
2. Results and Discussion
The molecular formula of compound 1 was determined to be C15H20O2 by HRMS. The IR spectrum exhibited a conjugated carbonyl group absorption at 1,695 cm−1. The 13C-NMR and HSQC spectra indicated the presence of two methyl, five methylene, four methine, and four quaternary carbon signals. The 1H-NMR spectrum exhibited the presence of a trisubstituted olefin and an exomethylene, as well as an aldehyde (Table 1).

Table 1.
NMR data for compounds 1 and 2 (500 MHz for 1H and 125 MHz for 13C in C6D6).
| position | 13C (ppm) | 1H (ppm) | |
|---|---|---|---|
| 1 | 1 | 2 | |
| 1 | 32.7 | 1.72–1.81 (2H, m) | 1.60–1.68 (m) |
| - | 1.91 (td, J = 12.2, 5.2 Hz) | ||
| 2 | 26.4 | 1.02–1.08 (m) | 0.94–1.01 (m) |
| 1.37–1.42 (m) | 1.43–1.50 (m) | ||
| 3 | 30.4 | 0.98–1.05 (m) | 0.98–1.06 (m) |
| 1.08–1.13 (m) | 1.09–1.15 (m) | ||
| 4 | 43.5 | 1.00–1.05 (m) | 1.50–1.55 (m) |
| 5 | 39.7 | - | - |
| 6 | 41.9 | 1.67 (dd, J = 13.0, 5.6 Hz) | 1.62 (t, J = 13.7 Hz) |
| 1.71 (dd, J = 13.4, 13.0 Hz) | 1.73 (dd, J = 13.7, 4.6 Hz) | ||
| 7 | 42.8 | 3.60 (dd, J = 13.4, 5.6 Hz) | 3.64 (dd, J = 13.7, 4.6 Hz) |
| 8 | 195.5 | - | - |
| 9 | 124.3 | 5.76 (d, J = 1.7 Hz) | 5.80 (d, J = 1.3 Hz) |
| 10 | 168.0 | - | - |
| 11 | 149.3 | - | - |
| 12 | 192.8 | 9.34 (s) | 9.31 (s) |
| 13 | 134.4 | 5.52 (s) | 5.52 (s) |
| 5.80 (s) | 5.88 (s) | ||
| 14 | 15.5 | 0.71 (s) | 0.63 (s) |
| 15 | 15.1 | 0.54 (d, J = 6.4 Hz) | 0.60 (d, J = 6.9 Hz) |
The HMBC spectrum of compound 1 showed correlations between H-15 and C-3, C-4, and C-5, between H-14 and C-4, C-5, C-6, and C-10, between H-7 and C-11 and C-13, and between H-9 and C-1, C-5, and C-7 (Figure 2). 1H-1H COSY correlations as shown in Figure 2 were also detected.
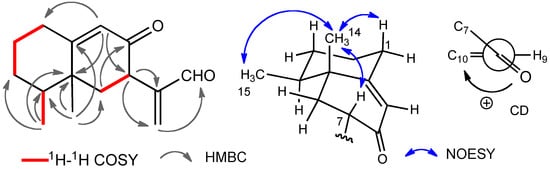
Figure 2.
Selected 2D correlations and the sign of the CD for compound 1.
From these results the planar structure was determined to be 8-oxoeremophila-9,11(13)-dien-12-al. The stereochemistry was revealed by the NOESY spectrum. NOEs between H-14 and H-1β, H-15, and H-7 were observed, therefore, H-7 was established to be β-oriented. The CD spectrum of compound 1 showed the Cotton effect [θ] +27000 at 237 nm (EtOH) [] which was similar to that of the known compound, 7S-eremophila-9,11-dien-8-one (5), also found in this extract (Figure 3). The structure of 1 was established to be 4S,5R,7S-8-oxoeremophila-9,11(13)-dien-12-al.
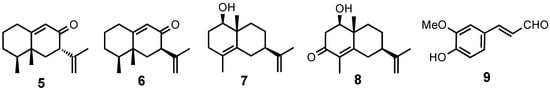
Figure 3.
Known compounds isolated in this work.
The molecular formula of compound 2 was the same as that of compound 1. The presence of a conjugated carbonyl group was also shown by the IR spectrum (1,693 cm−1). The 1H-NMR spectrum, which was very similar to that of compound 1, exhibited the presence of an aldehyde, a trisubstituted olefin, an exomethylene, a singlet methyl, and a doublet methyl (Table 1). 1H-1H COSY correlations from H-1 through H-4 to H-15 was detected and the planar structure was determined to be as depicted in the formula (Figure 4).
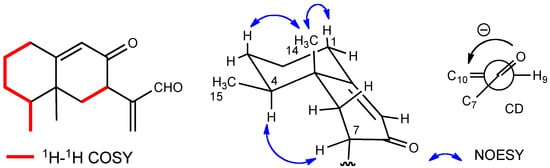
Figure 4.
Selected 2D correlations and the sign of the CD for compound 2.
The stereochemistry was deduced by the NOEs between H-14 and H-1β and H-3β and between H-4α and H-7α. The CD spectrum showed the Cotton effect [θ] -12000 at 225 nm (EtOH) which was similar to that of the known compound, 7R-eremophila-9,11-dien-8-one (6), also present in this extract (Figure 3). Therefore, compound 2 was established to be 4S,5R,7R-8-oxoeremophila-9,11(13)-dien-12-al.
Compound 3 exhibited a quasi-molecular ion peak at m/z 319 and the molecular formula was determined to be C20H30O3 by HRMS. The 1H-NMR spectrum indicated the presence of three doublet methyls, one singlet methyl, an exomethylene, a trisubstituted olefin, as well as two oxymethylene protons (Table 2).

Table 2.
NMR data for compounds 3 and 4 (500 MHz for 1H and 125 MHz for 13C; in C6D6).
| position | 13C (ppm) | 1H (ppm) | ||
|---|---|---|---|---|
| 3 | 4 | 3 | 4 | |
| 1 | 32.6 | 33.1 | 1.74–1.83 (m) | 1.76 (ddt, J = 14.6, 4.2, 2.0 Hz) |
| - | 1.83 (ddd, J = 14.6, 12.8, 5.2 Hz) | |||
| 2 | 26.5 | 26.7 | 1.03–1.09 (m) | 1.03–1.09 (m) |
| 1.38–1.43 (m) | 1.39–1.44 (m) | |||
| 3 | 30.4 | 30.5 | 1.00–1.05 (m) | 0.99–1.06 (m) |
| 1.10–1.15 (m) | 1.12–1.15 (m) | |||
| 4 | 43.6 | 43.1 | 1.00–1.05 (m) | 0.96–1.02 (m) |
| 5 | 39.6 | 38.8 | - | - |
| 6 | 41.7 | 35.7 | 1.72 (dd, J = 12.9, 4.1 Hz) | 1.27 (ddd, J = 14.4, 13.4, 4.4 Hz) |
| 1.85 (dd, J = 12.9, 4.1 Hz) | 1.45 (ddd, J = 13.4, 4.9, 3.5 Hz) | |||
| 7 | 47.5 | 34.3 | 3.12 (dd, J = 14.7, 4.1 Hz) | 2.14 (ddd, J = 16.6, 14.4, 4.9 Hz) |
| - | 2.25 (ddd, J = 16.6, 4.4, 3.5 Hz) | |||
| 8 | 196.7 | 197.3 | - | - |
| 9 | 124.4 | 124.7 | 5.73 (s) | 5.80 (s) |
| 10 | 168.0 | 168.5 | - | - |
| 11 | 143.9 | - | - | - |
| 12 | 66.3 | - | 4.98 (s) | - |
| 13 | 114.0 | - | 4.98 (s) | - |
| 5.36 (s) | - | |||
| 14 | 15.4 | 15.6 | 0.69 (s) | 0.61 (s) |
| 15 | 15.1 | 15.2 | 0.57 (d, J = 6.1 Hz) | 0.55 (d, J = 6.4 Hz) |
| 1' | 172.0 | - | - | - |
| 2' | 43.4 | - | 2.07 (d, J = 6.1 Hz) | - |
| 3' | 25.8 | - | 2.05–2.15 (m) | - |
| 4' 5' | 22.5 (2C) | - | 0.84 (d, J = 6.4 Hz) | - |
The presence of two carbonyl and two olefins was supported by the 13C-NMR spectrum. Therefore, this molecule should be bicyclic, because the degree of unsaturation was six and the number of the double bonds was four. The HMBC spectrum showed the correlations between H-15 and C-3, C-4, and C-5, between H-14 and C-4, C-5, C-6, and C-10, between H-7 and C-11, C-12, and C-13, between H-12 and C-1’, and other correlations shown in Figure 4. These observations indicate that this compound has an eremophilane skeleton and that 3-methylbutyryloxy group is substituted at C-12. The stereochemistry was determined by the correlations shown in Figure 4. The absolute configuration was also determined by the CD absorption of [θ] +43000 (235 nm in EtOH), which was almost the same as that of compound 1.
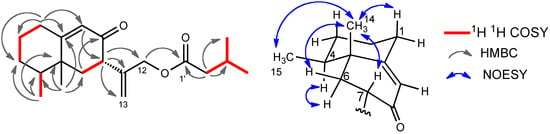
Figure 4.
Selected 2D correlations for compound 3.
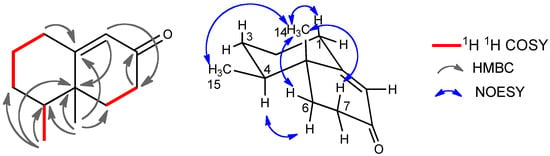
Figure 5.
Selected 2D correlations for compound 4.
The molecular formula of compound 4 was determined to be C12H18O by HRMS. The IR spectrum exhibited the absorption at 1,680 cm−1 for a conjugated ketone, which was supported by the NMR signals (δC 197.3, 168.5, 124.7; δH 5.80) (Table 2). The 13C-NMR spectrum showed the presence of only 12 carbon signals, including two methyl, five methylene, two methine, and three quaternary carbons. The HMBC and 1H-1H COSY correlations (Figure 5) clearly indicated the trinor-eremophilane skeleton. The NOESY spectrum showed that both methyl groups were β-oriented, and the absolute configuration was determined by the CD absorption as depicted in the formula. Compound 4 was established to be 4S,5R-trinoreremophil-9-en-8-one. This compound has been known as a synthetic intermediate as a chiral compound ([α]D +185.6 (CHCl3)) [], which also supported the absolute configuration of compound 4 (vide supra).
3. Experimental
General
Specific rotations and CD spectra were measured on a JASCO P-1030 and a JASCO J-725 auto recording polarimeter; IR spectra, on a Shimadzu FT/IR-8400S spectrophotometer; 1H and 13C NMR spectra (500 MHz and 125 MHz, respectively), on a Varian 500-MR spectrometer. Mass spectra, including high-resolution ones, were recorded on a JEOL JMS-700 MStation. A Chemcopak Nucleosil 50-5 column (4.6 × 250 mm) and a hexane-ethyl acetate solvent system were used for HPLC (JASCO pump system). Silica gel BW127ZH (100–270 mesh, Fuji Silysia) was used for column chromatography. Silica gel 60 F254 plates (Merck) were used for TLC.
Sample 1 (200982) was collected in the boundary between Luhuo and Seda Counties, Sichuan (N 31°42'30.2'', E 100°42'31.6''; altitude 3,700 m) and sample 2 (200938) in Litang County, Sichuan (N: 30°13'32'', E:100°16'3.6''; altitude 4,400 m) in 2009 (voucher specimens No. 200982 and 200938, were deposited in the Herbarium of Kunming Institute of Botany). Both samples were identified by X. Gong, one of the authors.
Sample 1 (200982; dried weight 21.1 g) was extracted with EtOAc to give an extract (424 mg), which was separated by column chromatography (n-hexane-EtOAc gradient) followed by HPLC (Nucleosil 50-5, n-hexane-EtOAc) to afford 1 (0.6 mg), 2 (0.3 mg), a mixture of 7S- and 7R-eremophila-9,11-dien-8-one (5 and 6, 60 mg), and 4-hydroxy-3-methoxycinnamaldehyde (9, 0.2 mg).
Sample 2 (200938; dried weight 42.1 g) was extracted with EtOAc to give an extract (1.3 g), which was separated by column chromatography (n-hexane-EtOAc gradient) followed by HPLC (Nucleosil 50-5, n-hexane-EtOAc) to afford 3 (2.4 mg), 4 (2.1 mg), eudesma-4,11-dien-1β-ol (7, 1.1 mg), 1β-hydroxyeudesma-4,11-dien-3-one (8, 1.4 mg), 4-hydroxy-3-methoxycinnamaldehyde (9, 0.2 mg), and vanillin (0.2 mg) (Scheme 2).
4S,5R,7S-8-Oxoeremophila-9,11(13)-dien-12-al (1): [α]D22 +99.5 (c 0.12, EtOH); FT-IR (KBr) 1695, 1676 cm−1; CD [θ] (EtOH) -5323 (319 nm), +27023 (237 nm); MS (CI) m/z 232 [M]+, 150, 135 (base); HRMS (CI) Obs m/z 232.1456 (Calcd for C15H20O2 232.1463).
4S,5R,7R-8-Oxoeremophila-9,11(13)-dien-12-al (2): [α]D22 −72 (c 0.01, EtOH); FT-IR (KBr) 1693, 1679 cm−1; CD [θ] (EtOH) +667 (328 nm), −12025 (225 nm); MS (CI) m/z 233 [M+H]+ (base), 150, 135; HRMS (CI) Obs m/z 233.1540 (Calcd for C15H21O2 233.1541).
4S,5R,7S-12-(3′-Methylbutyryloxy)eremophila-9,11(13)-dien-8-one (3): [α]D21 +178 (c 0.24, EtOH); FTIR (KBr) 1737, 1677 cm−1; CD [θ] (EtOH) −7240 (319 nm), +43635 (235 nm); MS (CI) m/z 319 [M+H]+ (base), 217; HRMS (CI) Obs m/z 319.2275 (Calcd for C20H31O3 319.2273).
4S,5R-Trinoreremophil-9-en-8-one (4): [α]D22 +143 (c 0.14, EtOH); FTIR (KBr) 1680 cm−1; CD [θ] (EtOH) -2768 (319 nm), +34310 (238 nm); MS (CI) m/z 179 [M+H]+, 89, 61 (base); HRMS (CI) Obs m/z 179.1422 (Calcd for C12H19O 179.1436).
4. Conclusions
Sample 1 afforded compounds 1 and 2, and sample 2 compounds 3 and 4. This is the first report of the isolation of eremophilane-type sesquiterpenoids from Cremanthodium spp. A trinoreremophilane compound, dendryphilellin A, has been isolated from the marine deuteromycete Dendryphiella salina [], but there are only a few examples of simple trinoreremophilanes reported so far [,,,], which are biogenetically closely related with compounds 1, 2, 5, or 6. The present results show that eremophilane-type sesquiterpenoids are common compounds both in Ligularia and Cremanthodium, implying that the genus Cremanthodium is quite close to Ligularia or Parasenecio [,,]. More samples are going to be investigated in the near future.
Acknowledgments
We thank Mrs. Guowen Hu of Kunming Institute of Botany for research coordination. This work was partly supported by a Grant-in-Aid for Scientific Research from JSPS (No. 21404009).
References and Notes
- Liu, J.; Liu, S.; Ho, T.; Lu, A. Karyological studies on the Sino-Himalayan genus, Cremanthodium (Asteraceae: Senecionae). Bot. J. Linn. Soc. 2001, 135, 107–112. [Google Scholar]
- Liu, J.Q.; Wang, Y.J.; Wang, A.L.; Ohba, H.; Abbott, R.J. Radiation and diversification within the Ligularia-Cremanthodium-Parasenecio complex (Asteraceae) triggered by uplift of the Qinghai-Tibetan plateau. Mol. Phylogenet. Evol. 2006, 38, 31–49. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Jia, Z.J. Lignan and a coumarin from Cremanthodium ellisii Kitam. Ind. J. Chem. 1995, 34B, 975–977. [Google Scholar]
- Chen, H.; Zhu, T.; Shen, X.M.; Jia, Z.J. Four new sesquiterpene polyol esters from Cremanthodium ellisii. J. Nat. Prod. 1996, 59, 1117–1120. [Google Scholar] [CrossRef]
- Chen, H.; Jia, Z.J.; Tan, R.X. Two new oplopanol esters from Cremanthodium ellisii. Planta Med. 1997, 63, 245–247. [Google Scholar] [CrossRef]
- Su, B.N.; Zhu, Q.X.; Jia, Z.J. Nor-lignan and sesquiterpenes from Cremanthodium ellisii. Phytochemistry 2000, 53, 1103–1108. [Google Scholar]
- Wang, A.X.; Zhang, Q.; Jia, Z.J. Phenylpropanosides, lignans and other constituents from Cremanthodium ellisii. Pharmazie 2004, 59, 889–892. [Google Scholar]
- Zhu, Y.; Yang, L.; Jia, Z.J. Novel highly oxygenated bisabolane sesquiterpenes from Cremanthodium discoideum. J. Nat. Prod. 1999, 62, 1479–1483. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Q.X.; Jia, Z.J. Epoxide sesquiterpenes and steroids from Cremanthodium discoideum. Aust. J. Chem. 2000, 53, 831–834. [Google Scholar] [CrossRef]
- Cremanthodium potaninii (triterpene): Yang, A.; Zeng, Y.; Yang, Z Isolation and structural elucidation of triterpenes from Cremanthodiium potaninii C. Winkl. Zhongchengyao 2010, 32, 636–639.
- Tu, Y.; Yang, R.; Shou, Q.; Wang, B.; Peng, T. Studies on chemical constituents of essential oils from Cremanthodium pleurocaule. Zhongguo Zhongyao Zazhi 2006, 31, 522–524. [Google Scholar]
- Hanai, R.; Gong, X.; Tori, M.; Kondo, S.; Otose, K.; Okamoto, Y.; Nishihama, T.; Murota, A.; Shen, Y.; Wu, S.; Kuroda, C. Chemical and Genetic Study of Ligularia tongolensis, Ligularia cymbulifera, and Ligularia atroviolacea in the Hengduan Mountains of China. Bull. Chem. Soc. Jpn. 2005, 78, 1302–1308. [Google Scholar] [CrossRef]
- Tori, M.; Honda, K.; Nakamizo, H.; Okamoto, Y.; Sakaoku, M.; Takaoka, S.; Gong, X.; Shen, Y.; Kuroda, C.; Hanai, R. Chemical constituents of Ligularia virgaurea and its diversity in southwestern Sichuan of China. Tetrahedron 2006, 62, 4988–4995. [Google Scholar]
- Tori, M.; Fujiwara, M.; Okamoto, Y.; Tanaka, M.; Gong, X.; Shen, Y.; Hanai, R.; Kuroda, C. New Oplopane-type Sesquiterpenoids from Ligularia duciformis. Nat. Prod. Commun. 2007, 2, 357–360. [Google Scholar]
- Tori, M.; Watanabe, A.; Matsuo, S.; Okamoto, Y.; Tachikawa, K.; Takaoka, S.; Gong, X.; Kuroda, C.; Hanai, R. Diversity of Ligularia kanaitzensis in sesquiterpenoid composition and neutral DNA sequences. Tetrahedron 2008, 64, 4486–4495. [Google Scholar] [CrossRef]
- Tori, M.; Okamoto, Y.; Tachikawa, K.; Mihara, K.; Watanabe, A.; Sakaoku, M.; Takaoka, S.; Tanaka, M.; Gong, X.; Kuroda, C.; Hattori, M.; Hanai, R. Isolation of new eremophilane-type sesquiterpenoids, subspicatins A–D and subspicatolide from Ligularia subspicata, and chemical and genetic diversity of the species. Tetrahedron 2008, 64, 9136–9142. [Google Scholar]
- Nagano, H.; Torihata, A.; Matsushima, M.; Hanai, R.; Saito, Y.; Baba, M.; Tanio, Y.; Okamoto, Y.; Takashima, Y.; Ichihara, M.; Gong, X.; Kuroda, C.; Tori, M. Chemical and Genetic Study of Ligularia cyathiceps in Yunnan Province of China. Helv. Chim. Acta 2009, 92, 2071–2081. [Google Scholar] [CrossRef]
- Saito, Y.; Hattori, M.; Iwamoto, Y.; Takashima, Y.; Mihara, K.; Sasaki, Y.; Fujiwara, M.; Sakaoku, M.; Shimizu, A.; Chao, X.; Kuroda, C.; Gong, X.; Hanai, R.; Tori, M. Overlapping chemical and genetic diversity in Ligularia lamarum and Ligularia subspicata. Isolation of ten new eremophilanes and a new seco-bakkane compound. Tetrahedron 2011, 67, 2220–2231. [Google Scholar] [CrossRef]
- Djerassi, C.; Rbcords, R.; Bunnenberg, E.; Mislow, K.; Moscowitz, A. Inherently dissymmetric chromophores. Optical rotatory dispersion of α,β-unsaturated ketones and conformational analysis of cyclohexenones. J. Am. Chem. Soc. 1962, 84, 870–872. [Google Scholar] [CrossRef]
- Paquette, L.A.; Wang, T.Z.; Philippo, M.G.; Wang, S. Total synthesis of the cembranoid diterpene lactone (+)-cleomeolide. Some remarkable conformational features of nine-membered belts linked in 2,6-fashion to a methylenecyclohexanone core. J. Am. Chem. Soc. 1994, 116, 3367–3374. [Google Scholar] [CrossRef]
- Guerriero, A.; D’Ambrosio, M.; Cuomo, V.; Vanzanella, F.; Pietra, F. Dendrophiellin A, the first fungal trinor-eremophilane. Isolation from the marine deuteromycete Dendryphiella salina (SUTHERLAND) PUGH et NICOT. Helv. Chim. Acta 1988, 71, 57–61. [Google Scholar] [CrossRef]
- Maurer, B.; Frachenboud, M.; Grieder, A.; Ohloff, G. Zur Kenntnis der sesquiterpenoiden C12-Ketone des ätherichen Öls von Vetiveria zizanioides (L.) Nash. Helv. Chim. Acta 1972, 55, 2371–2382. [Google Scholar] [CrossRef]
- Hikino, H.; Hikino, Y.; Koakutsu, S.; Takemoto, T. Structure and absolute configuration of narchinol A. Phytochemistry 1972, 11, 2097–2099. [Google Scholar]
- Bohlmann, F.; Kramp, W.; Robinson, H.; King, R.M. A norsequiterpene from Senecio humillimus. Phytochemistry 1981, 20, 1739–1740. [Google Scholar]
- Zdero, C.; Bohlmann, F. Eremophilanolides, eudesmanolides, guaianolides and other constituents from Ondetia lineari. Phytochemistry 1989, 28, 1653–1660. [Google Scholar] [CrossRef]
- Saito, Y.; Ichihara, M.; Okamoto, Y.; Gong, X.; Kuroda, C.; Tori, M. Five new subspicatins and noreremophilane from Parasenecio petasitoides collected in China. Tetrahedron Lett. 2011, 52, 6388–6391 and references cited therein. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–9 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).