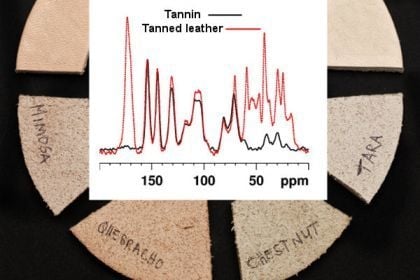
Tannin Fingerprinting in Vegetable Tanned Leather by Solid State NMR Spectroscopy and Comparison with Leathers Tanned by Other Processes
Abstract
1. Introduction
2. Results and Discussion
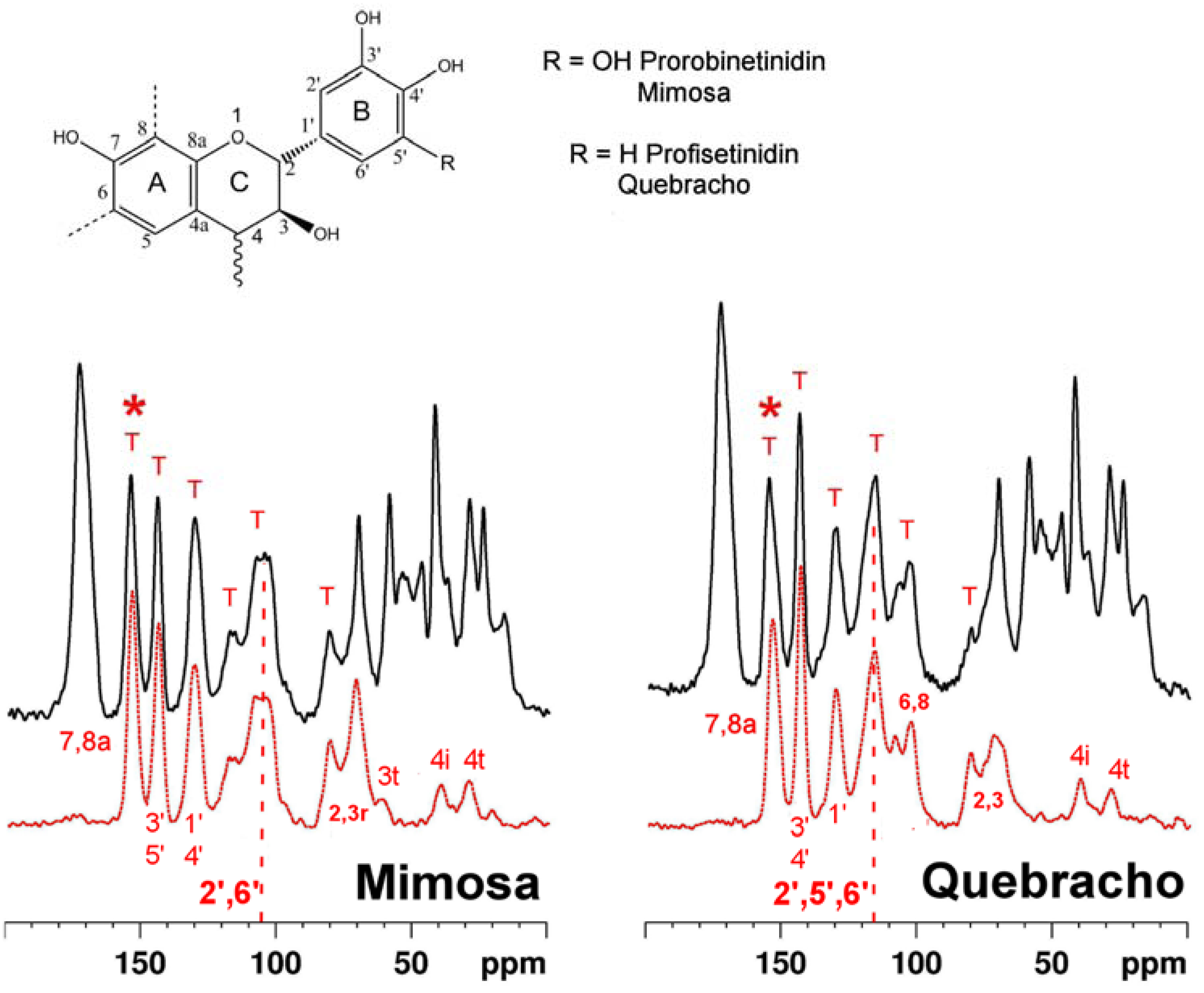
2.1. Tanning with condensed tannins
2.2. Tanning with hydrolyzable tannins

2.3. Chromium (III) tanning

2.4. Tanning with aluminium (III) and glutaraldehyde

3. Experimental Section
3.1. The tanning processes
3.2. Solid state NMR
4. Conclusions
Acknowledgements
References and Notes
- Covington, A.D. Modern tanning chemistry. Chem. Soc. Rev. 1997, 1997, 111–126. [Google Scholar] [CrossRef]
- Covington, A.D. Tanning Chemistry: The Science of Leather; RSC Publishing: Cambridge, UK, 2009. [Google Scholar]
- Ogrinc, N.; Kosir, I.J.; Spangenberg, J.E.; Kidric, J. The application of NMR and MS methods for detection of adulteration of wine, fruit juices, and olive oil. A review. Anal. Bioanal. Chem. 2003, 376, 424–430. [Google Scholar]
- Larsson, P.T. Interaction between cellulose I and hemicelluloses studied by spectral fitting of CP/MAS C-13-NMR spectra. Hemicelluloses: Sci. Technol. 2004, 864, 254–268. [Google Scholar]
- Bugay, D.E. Characterization of the solid-state: spectroscopic techniques. Advan. Drug Delivery Rev. 2001, 48, 43–65. [Google Scholar] [CrossRef]
- Wilson, M.A.; Hatcher, P.G. Detection of Tannins in Modern and Fossil Barks and in Plant Residues by High-Resolution Solid-State C-13 Nuclear Magnetic-Resonance. Org. Geochem. 1988, 12, 539–546. [Google Scholar] [CrossRef]
- Gamble, G.R.; Akin, D.E.; Makkar, H.P.S.; Becker, K. Biological degradation of tannins in sericea lespedeza (Lespedeza cuneta) by the white rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus analyzed by solid-state C-13 nuclear magnetic resonance spectroscopy. Appl. Environ. Microbiol. 1996, 62, 3600–3604. [Google Scholar]
- Lorenz, K.; Preston, C.M. Characterization of high-tannin fractions from humus by carbon-13 cross-polarization and magic-angle spinning nuclear magnetic resonance. J. Environ. Qual. 2002, 31, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, K.; Preston, C.M.; Raspe, S.; Morrison, I.K.; Feger, K.H. Litter decomposition and humus characteristics in Canadian and German spruce ecosystems: information from tannin analysis and C-13 CPMAS NMR. Soil Biol. Biochem. 2000, 32, 779–792. [Google Scholar]
- Hoong, Y.B.; Pizzi, A.; Md. Tahir, P.; Pasch, H. Characterization of Acacia mangium polyflavonoid tannins by MALDI-TOF mass spectrometry and CP-MAS 13C NMR. Eur. Polym. J. 2010, 46, 1268–1277. [Google Scholar]
- Navarrete, P.; Pizzi, A.; Pasch, H.; Rode, K.; Delmotte, L. MALDI-TOF and 13C NMR characterization of maritime pine industrial tannin extract. Ind. Crops Prod. 2010, 32, 105–110. [Google Scholar]
- Yu, Z.; Dahlgren, R.A. Evaluation of methods for measuring polyphenols in conifer foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar]
- Smernik, R.J.; Oades, J.M. Solid-state C-13-NMR dipolar dephasing experiments for quantifying protonated and non-protonated carbon in soil organic matter and model systems. Eur. J. Soil Sci. 2001, 52, 103–120. [Google Scholar] [CrossRef]
- Moubarik, A.; Pizzi, A.; Allal, A.; Charrier, F.; Charrier, B. Cornstarch and tannin in phenol-formaldehyde resins for plywood production. Ind. Crops Prod. 2009, 30, 188–193. [Google Scholar]
- Moubarik, A.; Charrier, B.; Allal, A.; Charrier, F.; Pizzi, A. Development and optimization of a new formaldehyde-free cornstarch and tannin wood adhesive. Eur. J. Wood Wood Prod. 2010, 68, 167–177. [Google Scholar]
- Pichelin, F.; Nakatani, M.; Pizzi, A.; Wieland, S.; Despres, A.; Rigolet, S. Structural beams from thick wood panels bonded industrially with formaldehyde-free tannin adhesives. Forest Prod. J. 2006, 56, 31–36. [Google Scholar]
- Grigsby, W.J.; Hill, S.J.; McIntosh, C.D. NMR estimation of extractables from bark: Analysis method for quantifying tannin extraction from bark. J. Wood Chem. Technol. 2003, 23, 179–195. [Google Scholar] [CrossRef]
- Odlyha, M.; Cohen, N.S.; Foster, G.M.; Aliev, A.; Verdonck, E.; Grandy, D. Dynamic mechanical analysis (DMA), C-13 solid state NMR and micro-thermomechanical studies of historical parchment. J. Therm. Anal. Calorim. 2003, 71, 939–950. [Google Scholar] [CrossRef]
- Simon, C.; Pizzi, A. Lightfast and high shrinkage temperature leather produced using vegetable tannins and novel melamine-urea-formaldehyde tanning formulations. J. Amer. Leather Chem. Assoc. 2003, 98, 83–96. [Google Scholar]
- Bardet, M.; Gerbaud, G.; Le Pape, L.; Hediger, S.; Tran, Q.K.; Boumlil, N. Nuclear magnetic resonance and electron paramagnetic resonance as analytical tools to investigate structural features of archaeological leathers. Anal. Chem. 2009, 81, 1505–11. [Google Scholar]
- Brown, E.M.; Dudley, R.L.; Elsetinow, A.R. A conformational study of collagen as affected by tanning procedures. J. Amer. Leather Chem. Assoc. 1997, 92, 225–232. [Google Scholar]
- Brown, E.M.; Dudley, R.L. Approach to a tanning mechanism: Study of the interaction of aluminum sulfate with collagen. J. Amer. Leather Chem. Assoc. 2005, 100, 401–409. [Google Scholar]
- Ayres, M.P.; Clausen, T.P.; MacLean, S.F.; Redman, A.M.; Reichardt, P.B. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
- Czochanska, Z.; Foo, L.Y.; Newman, R.H.; Porter, L.J. Polymeric Proanthocyanidins - Stereochemistry, Structural Units, and Molecular-Weight. J. Chem. Soc.-Perkin Trans. 1 1980, 2278–2286. [Google Scholar]
- Tarascou, I.; Barathieu, K.; Simon, C.; Ducasse, M.A.; Andre, Y.; Fouquet, E.; Dufourc, E.J.; de Freitas, V.; Laguerre, M.; Pianet, I. A 3D structural and conformational study of procyanidin dimers in water and hydro-alcoholic media as viewed by NMR and molecular modeling. Magn. Resonance Chem. 2006, 44, 868–880. [Google Scholar] [CrossRef]
- Davis, A.L.; Cai, Y.; Davies, A.P.; Lewis, J.R. H-1 and C-13 NMR assignments of some green tea polyphenols. Magn. Resonance Chem. 1996, 34, 887–890. [Google Scholar]
- Pasch, H.; Pizzi, A.; Rode, K. MALDI-TOF mass spectrometry of polyflavonoid tannins. Polymer 2001, 42, 7531–7539. [Google Scholar]
- Covington, A.D.; Lilley, T.H.; Song, L.; Evans, C.S. Collagen and polyphenols: New relationships and new outcomes. Part 1. Flavonoid reactions for new tanning processes. J. Amer. Leather Chem. Assoc. 2005, 100, 325–335. [Google Scholar]
- Covington, A.D. Vegetable tanning. In Tanning Chemistry: the Science of Leather; RSC Publishing: Cambridge, UK, 2009; pp. 281–314. [Google Scholar]
- Galvez, J.M.G.; Riedl, B.; Conner, A.H. Analytical studies on tara tannins. Holzforschung 1997, 51, 235–243. [Google Scholar] [CrossRef]
- Pizzi, A.; Pasch, H.; Rode, K.; Giovando, S. Polymer Structure of Commercial Hydrolyzable Tannins by Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight Mass Spectrometry. J. Appl. Polym. Sci. 2009, 113, 3847–3859. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Synthesis and spectroscopic characterisation of the polygalloyl esters of polyols-models for gallotannins. J. Soc. Leather Technol. Chem. 2003, 87, 179–188. [Google Scholar]
- Orabi, M.A.A.; Taniguchi, S.; Yoshimura, M.; Yoshida, T.; Kishino, K.; Sakagami, H.; Hatano, T. Hydrolyzable Tannins of Tamaricaceous Plants. III. Hellinoyl- and Macrocyclic-Type Ellagitannins from Tamarix nilotica. J. Nat. Prod. 2010, 73, 870–879. [Google Scholar] [CrossRef]
- Magid, A.A.; Voutquenne-Nazabadioko, L.; Harakat, D.; Moretti, C.; Lavaud, C. Phenolic glycosides from the stem bark of Caryocar villosum and C-glabrum. J. Nat. Prod. 2008, 71, 914–917. [Google Scholar] [CrossRef]
- Duer, M.J. Introduction to Solid-state NMR Spectroscopy; Blackwell Science: Oxford, UK, 2004; pp. 116–150. [Google Scholar]
- Aliev, A.E. Solid-state NMR studies of collagen-based parchments and gelatin. Biopolymers 2005, 77, 230–245. [Google Scholar] [CrossRef]
- Dwek, R.A. NMR in Biochemistry; Clarendon: Oxford, UK, 1973. [Google Scholar]
- Covington, A.D.; Hancock, R.A.; Ioannidis, I.A. Mechanistic Studies of Mineral Tanning .1. Solid-State Al-27 Nmr-Studies and the Thermodynamics of Shrinking. J. Soc. Leather Technol. Chem. 1989, 73, 1–8. [Google Scholar]
- Martin, R.W.; Zilm, K.W. Preparation of protein nanocrystals and their characterization by solid state NMR. J. Magn. Resonance 2003, 165, 162–174. [Google Scholar] [CrossRef]
- Haraguchi, H.; Fujiwara, S. Aluminum complexes in solution as studied by aluminum-27 nuclear magnetic resonance. J. Phys. Chem. 1969, 73, 3467–3473. [Google Scholar] [CrossRef]
- de Lacaillerie, J.B.D.; Fretigny, C.; Massiot, D. MAS NMR spectra of quadrupolar nuclei in disordered solids: The Czjzek model. J. Magn. Resonance 2008, 192, 244–251. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Romer, F.H.; Underwood, A.P.; Senekal, N.D.; Bonnet, S.L.; Duer, M.J.; Reid, D.G.; Van der Westhuizen, J.H. Tannin Fingerprinting in Vegetable Tanned Leather by Solid State NMR Spectroscopy and Comparison with Leathers Tanned by Other Processes. Molecules 2011, 16, 1240-1252. https://doi.org/10.3390/molecules16021240
Romer FH, Underwood AP, Senekal ND, Bonnet SL, Duer MJ, Reid DG, Van der Westhuizen JH. Tannin Fingerprinting in Vegetable Tanned Leather by Solid State NMR Spectroscopy and Comparison with Leathers Tanned by Other Processes. Molecules. 2011; 16(2):1240-1252. https://doi.org/10.3390/molecules16021240
Chicago/Turabian StyleRomer, Frederik H., Andrew P. Underwood, Nadine D. Senekal, Susan L. Bonnet, Melinda J. Duer, David G. Reid, and Jan H. Van der Westhuizen. 2011. "Tannin Fingerprinting in Vegetable Tanned Leather by Solid State NMR Spectroscopy and Comparison with Leathers Tanned by Other Processes" Molecules 16, no. 2: 1240-1252. https://doi.org/10.3390/molecules16021240
APA StyleRomer, F. H., Underwood, A. P., Senekal, N. D., Bonnet, S. L., Duer, M. J., Reid, D. G., & Van der Westhuizen, J. H. (2011). Tannin Fingerprinting in Vegetable Tanned Leather by Solid State NMR Spectroscopy and Comparison with Leathers Tanned by Other Processes. Molecules, 16(2), 1240-1252. https://doi.org/10.3390/molecules16021240