Effect of End Groups on the Raman Spectra of Lycopene and β-Carotene under High Pressure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of end groups on Raman spectra of carotenoids at ambient conditions


 is the diabatic frequency of the ith vibration mode in the S0 state at normal pressure,
is the diabatic frequency of the ith vibration mode in the S0 state at normal pressure,  is the increment of
is the increment of  as a result of the shortened bond lengths induced by high pressure,
as a result of the shortened bond lengths induced by high pressure,  is the contribution of vibronic coupling. Consequently, the pressure induced increase of the adiabatic frequency
is the contribution of vibronic coupling. Consequently, the pressure induced increase of the adiabatic frequency  can be expressed as:
can be expressed as:

 is the increment of
is the increment of  . It has been shown that stronger vibronic coupling between the S0 and S1 states can result in a more obvious solvent effect on the ν1 frequency [19,20]. For these reasons, the larger difference between ν1 frequency in n-hexane and CS2 for β-carotene than that for lycopene suggests that the vibronic coupling between the S1 and S0 states of β-carotene is stronger than that of lycopene.
. It has been shown that stronger vibronic coupling between the S0 and S1 states can result in a more obvious solvent effect on the ν1 frequency [19,20]. For these reasons, the larger difference between ν1 frequency in n-hexane and CS2 for β-carotene than that for lycopene suggests that the vibronic coupling between the S1 and S0 states of β-carotene is stronger than that of lycopene.2.2. Effects of end groups on Raman spectra of carotenoids under high pressure




 >
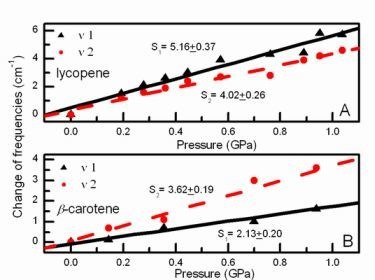
>  . In addition, Figure 3 indicates that with increasing pressure, vibronic coupling between S0 and S1 of lycopene is smaller than that of β-carotene, namely,
. In addition, Figure 3 indicates that with increasing pressure, vibronic coupling between S0 and S1 of lycopene is smaller than that of β-carotene, namely,  <
<  . Therefore:
. Therefore:

3. Experimental
General
4. Conclusions
Acknowledgements
References
- Frank, H.A.; Cogdell, R.J. Carotenoid in Photosynthesis. Photochem. Photobiol. 1996, 63, 257–264. [Google Scholar] [CrossRef]
- Polívka, T.; Sundström, V. Ultrafast Dynamics of Carotenoid Excited States-from Solution to Natural and Artificial Systems. Chem. Rev. 2004, 104, 2021–2072. [Google Scholar] [CrossRef]
- Coleman, W.F. Molecular Models of Lycopene and Other Carotenoids. J. Chem. Educ. 2008, 85, 320. [Google Scholar] [CrossRef]
- Fujiwara, M.; Sugisaki, M.; Gall, A.; Robert, B.; Cogdell, R.J.; Hashimoto, H. Ultrafast Optical Responses of β-carotene and Lycopene Probed by sub-20-fs Time-Resolved Coherent Spectroscopy. J. Lumin. 2009, 129, 1808–1812. [Google Scholar] [CrossRef]
- Nagae, H.; Kuki, M.; Zhang, J.P.; Sashima, T.; Mukai, Y.; Koyama, Y. Vibronic Coupling through the in-Phase, C=C Stretching Mode Plays a Major Role in the 2Ag- to 1Ag- Internal Conversion of all-trans-β-Carotene. J. Phys. Chem. A 2000, 104, 4155–4166. [Google Scholar]
- Tsen, K.T.; D. Tsen, S.W.; G. Kiang, J. Lycopene is more potent than beta carotene in the neutralization of singlet oxygen: role of energy transfer probed by ultrafast Raman spectroscopy. J. Biomed. Opt. 2006, 11, 064025. [Google Scholar] [CrossRef]
- Guo, W.H.; Tu, C.Y.; Hu, C.H. Cis-Trans Isomerizations of β-Carotene and Lycopene: A Theoretical Study. J. Phys. Chem. B 2008, 112, 12158–12167. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Christofilos, D.; Arvanitidis, I.; Arvanitidis, I.; Kourouklis, G. Raman Spectroscopy Forintracellular Monitoring of Carotenoid in Blakeslea Trispora. Appl. Biochem. Biotechnol. 2009, 159, 478–487. [Google Scholar] [CrossRef]
- de Oliveira, V.E.; Castro, H.V.; Edwards, H.G.M.; de Oliveira, L.F.C. Carotenes and Carotenoids in Natural Biological Samples: A Raman Spectroscopic Analysis. J. Raman spectrosc. 2010, 41, 642–650. [Google Scholar]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman Spectroscopy in Natural Ccarotenoid Analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef]
- Ellervee, A.; Linnanto, J.; Freiberg, A. Spectroscopic and Quantum Chemical Study of Pressure Effects on Solvated Chlorophyll. Chem. Phys. Lett. 2004, 394, 80–84. [Google Scholar] [CrossRef]
- Chang, H.C.; Chang, C.Y.; Su, J.C.; Chu, W.C.; Jiang, J.C.; Lin, S.H. Conformations of 1-Butyl-3-methylimidazolium Chloride Probed by High Pressure Raman Spectroscopy. Int. J. Mol. Sci. 2006, 7, 417–424. [Google Scholar] [CrossRef]
- Murli, C.; Song, Y. Pressure-Induced Polymerization of Acrylic Acid: A Raman Spectroscopic Study. J. Phys. Chem. B 2010, 114, 9744–9750. [Google Scholar] [CrossRef]
- Saito, S.; Tasumi, M. Normal-Coordinate Analysis of β-Carotene Isomers and Assignments of the Raman and Infrared Bands. J. Raman Spectrosc. 1983, 14, 310–321. [Google Scholar] [CrossRef]
- Weesie, R.J.; Merlin, J.C.; Lugtenburg, J.; Britton, G.; Jansen, F.J.H.M.; Cornard, J.P. Semiempirical and Raman Spectroscopic Studies of Carotenoids. Biospectroscopy 1999, 5, 19–33. [Google Scholar] [CrossRef]
- Rimai, L.; Heyde, M.E.; Gill, D. Vibrational Spectra of Some Carotenoids and Related Linear Polyenes. A Raman Spectroscopic Study. J. Am. Chem. Soc. 1973, 95, 4493–4501. [Google Scholar] [CrossRef]
- Auerbach, R.A.; Christensen, R.L.; Granville, M.F.; Kohler, B.E. Absorption and emission of 2,12-dimethyltridecahexaene. J. Chem. Phys. 1981, 74, 4–9. [Google Scholar] [CrossRef]
- Hashimoto, H.; Koyama, Y. The C=C stretching Raman lines of β-Carotene isomers in the S1 state as detected by pump-probe resonance raman spectroscopy. Chem. Phys. Lett. 1989, 154, 321–325. [Google Scholar] [CrossRef]
- Liu, W.L.; Zheng, Z.R.; Zhu, R.B.; Liu, Z.G.; Xu, D.P.; Yu, H.M. Effect of Pressure and Solvent on Raman Spectra of All-trans-β-Carotene. J. Phys. Chem. A 2007, 111, 10044–10049. [Google Scholar]
- Lee, S.A.; Chan, C.K.; Page, J.B.; Walker, C.T. Temperature-dependent resonance Raman profiles of β-carotene in carbon disulfide. J. Chem. Phys. 1986, 84, 2497–2502. [Google Scholar] [CrossRef]
- Piermarini, G.J.; Block, S.; Barnett, J.D.; Forman, R.A. Calibration of the Pressure Dependence of the R1 Ruby Fluorescence Line to 195 Kbar. J. Appl. Phys. 1975, 46, 2774–2780. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the lycopene is available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huo, M.-M.; Liu, W.-L.; Zheng, Z.-R.; Zhang, W.; Li, A.-H.; Xu, D.-P. Effect of End Groups on the Raman Spectra of Lycopene and β-Carotene under High Pressure. Molecules 2011, 16, 1973-1980. https://doi.org/10.3390/molecules16031973
Huo M-M, Liu W-L, Zheng Z-R, Zhang W, Li A-H, Xu D-P. Effect of End Groups on the Raman Spectra of Lycopene and β-Carotene under High Pressure. Molecules. 2011; 16(3):1973-1980. https://doi.org/10.3390/molecules16031973
Chicago/Turabian StyleHuo, Ming-Ming, Wei-Long Liu, Zhi-Ren Zheng, Wei Zhang, Ai-Hua Li, and Da-Peng Xu. 2011. "Effect of End Groups on the Raman Spectra of Lycopene and β-Carotene under High Pressure" Molecules 16, no. 3: 1973-1980. https://doi.org/10.3390/molecules16031973