Synthesis and Biological Evaluation of 4-Aroyl-6,7,8-Trimethoxyquinolines as a Novel Class of Anticancer Agents
Abstract
:1. Introduction
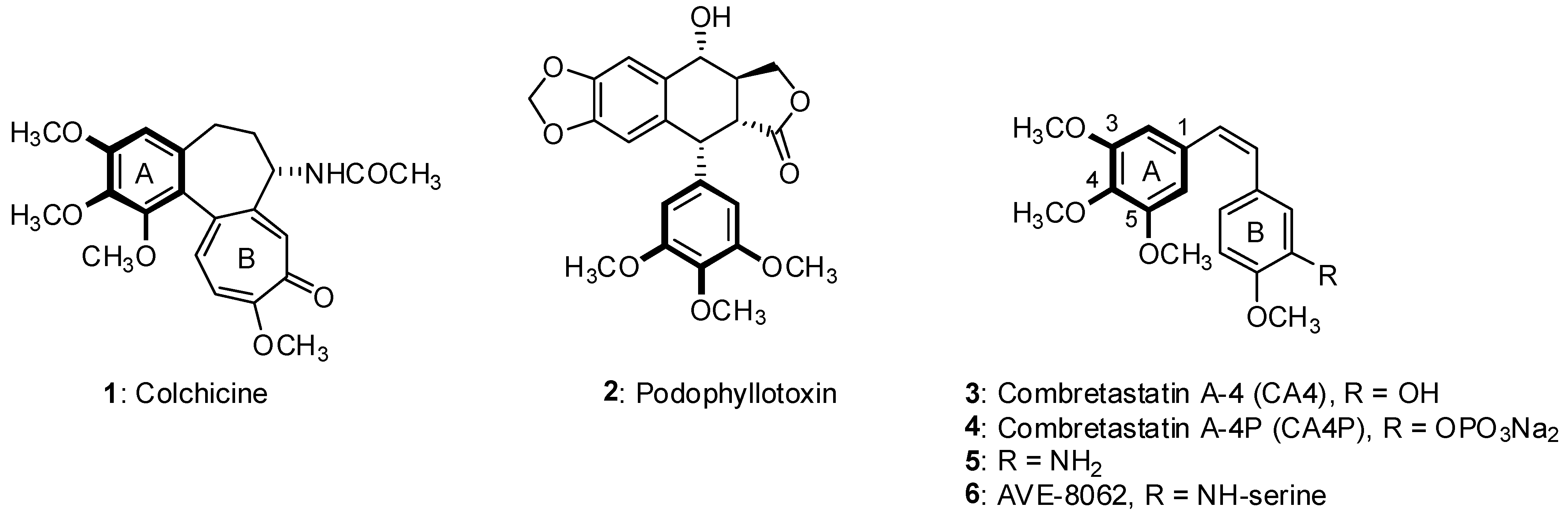
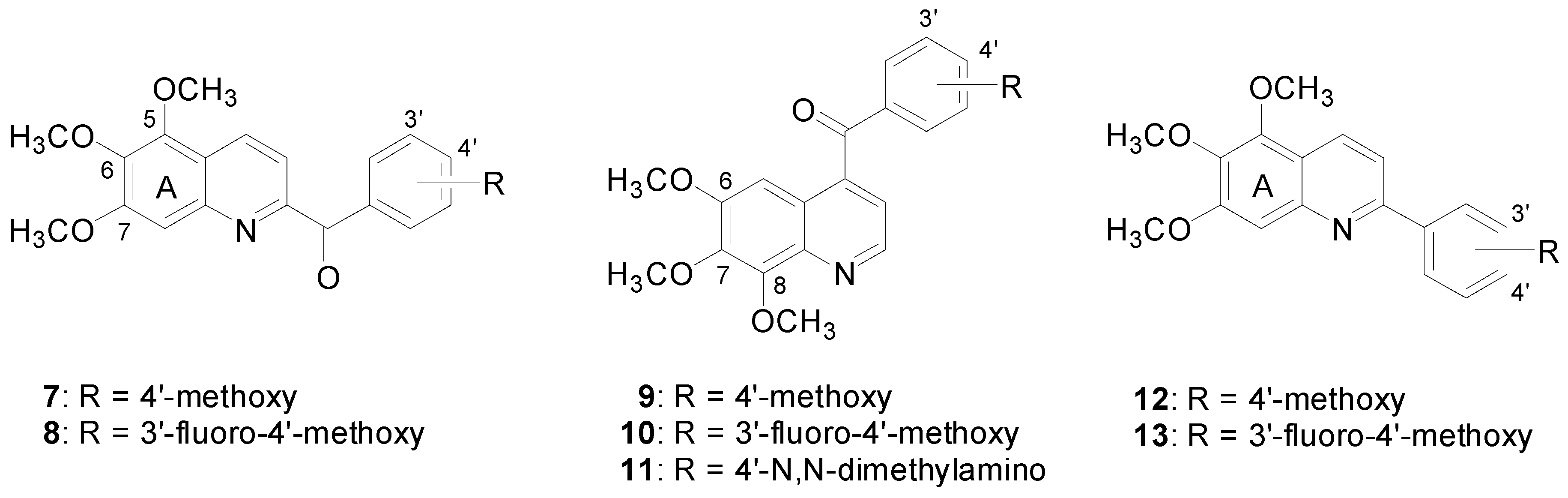
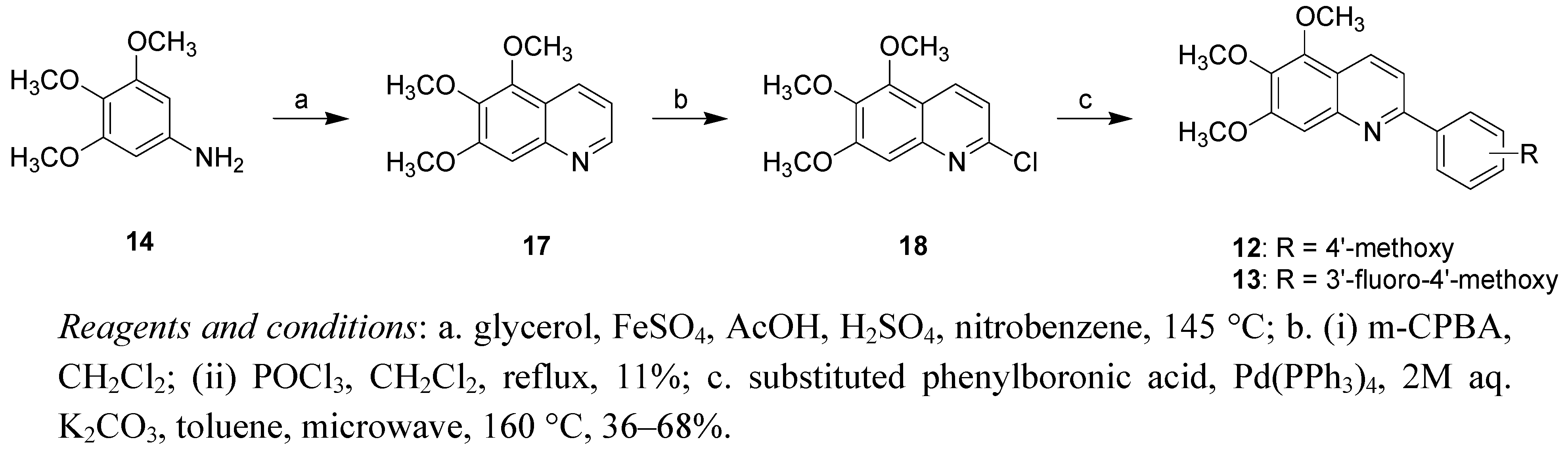
2. Results and Discussion

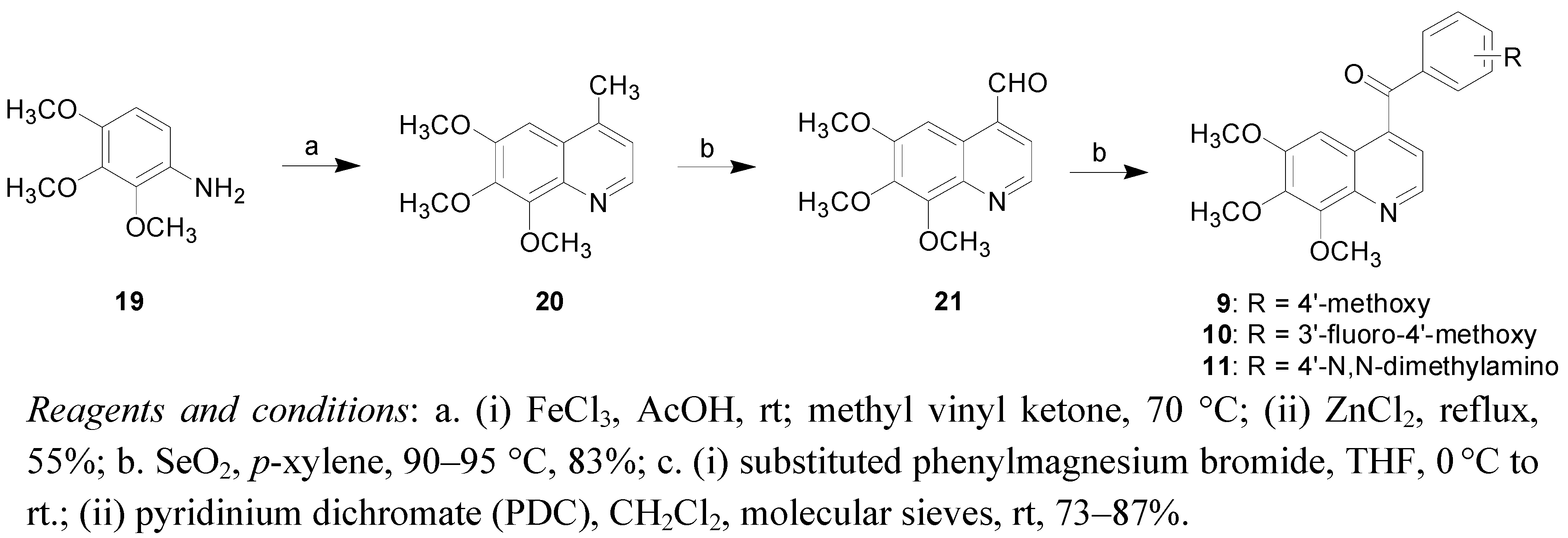
| Cell Type ( IC50 ± SDa, nM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd | KB | HT29 | MKN45 | ||||||
| 7 | > 5,000 | > 5,000 | > 5,000 | ||||||
| 8 | > 5,000 | > 5,000 | > 5,000 | ||||||
| 9 | 1,297 | ± | 371 | 2,092 | ± | 281 | 1,436 | ± | 272 |
| 10 | 1,597 | ± | 94 | 1,725 | ± | 530 | 1,047 | ± | 87 |
| 11 | 217 | ± | 39 | 327 | ± | 93 | 239 | ± | 47 |
| 12 | > 5,000 | > 5,000 > 5,000 | > 5,000 | ||||||
| > 5,000 | 14 | ||||||||
| > 5,000 | > 5,000 | ||||||||
| 13 | > 5,000 | > 5,000 > 5,000 | > 5,000 | ||||||
| > 5,000 | 15 | ||||||||
| > 5,000 | > 5,000 | ||||||||
| colchicine | 10.3 | ± | 0.9 | 16.2 | ± | 2.9 | 19.2 | ± | 1.0 |
| Cell lines | Resistant type | IC50 ± SDa | |||
|---|---|---|---|---|---|
| Vincristine, nMb | Paclitaxel, nMb | VP-16, µMb | 11, nM | ||
| KB | Parental | 0.4 ± 0.1 | 3.3 ± 1.2 | 1.1 ± 0.5 | 217.0 ± 39.0 |
| KB-VIN10 | MDR ↑ | 90.1 ± 7.4 | 16500 ± 707 | 23 ± 3 | 245.6 ± 8.5 |
| KB-S15 | MDR ↑ | 17.6 ± 2.2 | 273 ± 15 | 3.5 ± 0.3 | 213.3 ± 96.1 |
| KB-7D | MRP ↑ | 1.2 ± 0.4 | 7.9 ± 0.5 | 54 ± 3.5 | 252.3 ± 15.0 |
| Compd. | TubulinaIC50 ± SD (µM) | Colchicine bindingb (%) | |
|---|---|---|---|
| 1 µM | 5 µM | ||
| 11 | >10 | 37.6 | 45.6 |
| colchicine | 7.3 | 58.7 | 80.8 |
3. Experimental
3.1. General
3.2. Chemistry
3.2.1. General procedure for synthesis of compounds 7-11
3.3. Cell Growth Inhibitory Assay
3.4. Tubulin Polymerization in Vitro Assay [13,15]
3.5. Tubulin Competition-Binding Scintillation Proximity Assay [17,18,19]
4. Conclusions
Acknowledgements
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Li, Q.; Sham, H.L. Discovery and development of antimitotic agents that inhibit tubulin polymerisation for the treatment of cancer. Expert. Opin. Ther. Pat. 2002, 12, 1663–1702. [Google Scholar] [CrossRef]
- Kelleher, J.K. Tubulin binding affinities of podophyllotoxin and colchicine analogues. Mol. Pharmacol. 1976, 13, 232–241. [Google Scholar]
- Hamel, E. Antimitotic natural products and their interactions with tubulin. Med. Res. Rev. 1996, 16, 207–231. [Google Scholar] [CrossRef]
- Pettit, G.R.; Temple, C.J.; Narayanan, V.L.; Varma, R.; Simpson, M.J.; Boyd, M.R.; Rener, G.A.; Bansal, N. Antineoplastic agents 322. synthesis of combretastatin A-4 prodrugs. Anti-Cancer Drug Des 1995, 10, 299–309. [Google Scholar]
- Ohsumi, K.; Nakagawa, R.; Fukuda, Y.; Hatanaka, T.; Morinaga, Y.; Nihei, Y.; Ohishi, K.; Suga, Y.; Akiyama, Y.; Tsuji, T. Novel combretastatin analogues effective against murine solid tumors: design and structure-activity relationships. J. Med. Chem. 1998, 41, 3022–3032. [Google Scholar] [CrossRef]
- Liou, J.P.; Chang, C.W.; Song, J.S.; Yang, Y.N.; Yeh, C.F.; Tseng, H.Y.; Lo, Y.K.; Chang, Y.L.; Chang, C.M.; Hsieh, H.P. Synthesis and structure-activity relationship of 2-aminobenzophenone derivatives as antimitotic agents. J. Med. Chem. 2002, 45, 2556–2562. [Google Scholar] [CrossRef]
- Chuang, H.Y.; Chang, J.Y.; Lai, M.J.; Kuo, C.C.; Lee, H.Y.; Hsieh, H.P.; Chen, Y.J.; Chen, L.T.; Pan, W.Y.; Liou, J.P. 2-Amino-3,4,5-trimethoxybenzophenones as potent tubulin polymerization inhibitors. ChemMedChem 2011, 6, 450–456. [Google Scholar] [CrossRef]
- Nien, C.Y.; Chen, Y.C.; Kuo, C.C.; Hsieh, H.P.; Chang, C.Y.; Wu, J.S.; Wu, S.Y.; Liou, J.P.; Chang, J.Y. 5-Amino-2-aroylquinolines as highly potent tubulin polymerization inhibitors. J. Med. Chem. 2010, 53, 2309–2313. [Google Scholar]
- Liou, J.P.; Chang, J.Y.; Chang, C.W.; Chang, C.Y.; Mahindroo, N.; Kuo, F.M.; Hsieh, H.P. Synthesis and structure-activity relationships of 3-aminobenzophenones as antimitotic agents. J. Med. Chem. 2004, 47, 2897–2905. [Google Scholar] [CrossRef]
- Liou, J.P.; Hsu, K.S.; Kuo, C.C.; Chang, C.Y.; Chang, J.Y. A novel oral indoline-sulfonamide agent, J30, exhibits potent activity against human cancer cells in vitro and in vivo through the disruption of microtubule. J. Pharmacol. Exp. Ther. 2007, 323, 398–05. [Google Scholar] [CrossRef]
- Kuo, C.C.; Hsieh, H.P.; Pan, W.Y.; Chen, C.P.; Liou, J.P.; Lee, S.J.; Chang, Y.L.; Chen, L.T.; Chen, C.T.; Chang, J.Y. BPR0L075, a novel synthetic indole compound with antimitotic activity in human cancer cells, exerts effective antitumoral activity in vivo. Cancer Res. 2004, 64, 4621–4628. [Google Scholar] [CrossRef]
- Finlay, G.J.; Baguley, B.C.; Wilson, W.R. A semiautomated microculture method for investigating growth inhibitory effect of cytotoxic compounds on exponentially growing carcinoma cells. Anal. Biochem. 1984, 139, 272–277. [Google Scholar] [CrossRef]
- Liou, J.P.; Chang, J.Y.; Chang, C.W.; Chang, C.Y.; Mahindroo, N.; Kuo, F.M.; Hsieh, H.P. Synthesis and structure-activity relationships of 3-aminobenzophenones asantimitotic agents. J. Med. Chem. 2004, 47, 2897–2905. [Google Scholar] [CrossRef]
- Bollag, D.M.; McQueney, P.A.; Zhu, J.; Hensens, O.; Koupal, L.; Liesch, J.; Goetz, M.; Lazarides, E.; Woods, C.M. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995, 55, 2325–2333. [Google Scholar]
- Tahir, S.K.; Kovar, P.; Rosenberg, S.H.; Ng, S.C. Rapid colchicine competition-binding scintillation proximity assay using biotin-labeled tubulin. Biotechniques 2000, 29, 156–160. [Google Scholar]
- Tahir, S.K.; Han, E.K.; Credo, B.; Jae, H.S.; Pietenpol, J.A.; Scatena, C.D.; Wu-Wong, J.R.; Frost, D.; Sham, H.; Rosenberg, S.H.; Ng, S.C. A-204197, a new tubulin-binding agent with antimitotic activity in tumor cell lines resistant to known microtubule inhibitors. Cancer Res. 2001, 61, 5480–5485. [Google Scholar]
- Tahir, S.K.; Nukkala, M.A.; Zielinski Mozny, N.A.; Credo, R.B.; Warner, R.B.; Li, Q.; Woods, K.W.; Claiborne, A.; Gwaltney, S.L., II.; Frost, D.J.; Sham, H.L.; Rosenberg, S.H.; Ng, S.C. Biological activity of A-289099: An orally active tubulin-binding indolyloxazoline derivative. Mol. Cancer Ther. 2003, 2, 227–233. [Google Scholar]
- Sample Availability: Contact the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hsieh, C.-C.; Lee, H.-Y.; Nien, C.-Y.; Kuo, C.-C.; Chang, C.-Y.; Chang, J.-Y.; Liou, J.-P. Synthesis and Biological Evaluation of 4-Aroyl-6,7,8-Trimethoxyquinolines as a Novel Class of Anticancer Agents. Molecules 2011, 16, 2274-2284. https://doi.org/10.3390/molecules16032274
Hsieh C-C, Lee H-Y, Nien C-Y, Kuo C-C, Chang C-Y, Chang J-Y, Liou J-P. Synthesis and Biological Evaluation of 4-Aroyl-6,7,8-Trimethoxyquinolines as a Novel Class of Anticancer Agents. Molecules. 2011; 16(3):2274-2284. https://doi.org/10.3390/molecules16032274
Chicago/Turabian StyleHsieh, Cheng-Chih, Hsueh-Yun Lee, Chih-Ying Nien, Ching-Chuan Kuo, Chi-Yen Chang, Jang-Yang Chang, and Jing-Ping Liou. 2011. "Synthesis and Biological Evaluation of 4-Aroyl-6,7,8-Trimethoxyquinolines as a Novel Class of Anticancer Agents" Molecules 16, no. 3: 2274-2284. https://doi.org/10.3390/molecules16032274





