Fungicidal Properties of the Essential Oil of Hesperozygis marifolia on Aspergillus flavus Link
Abstract
:1. Introduction
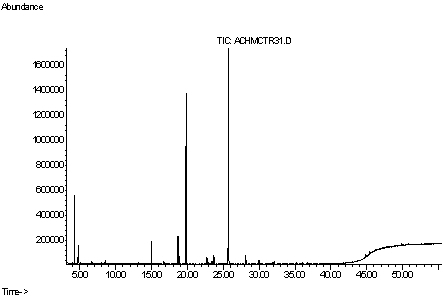
2. Results and Discussion
| Compound | Composition* (%) | Retention time (min) |
|---|---|---|
| α-Pinene | 0.16 | 3.92 |
| β-Pinene | 0.12 | 5.74 |
| Limonene | 0.41 | 8.52 |
| Cyclohexanone, 3-methyl- | 0.16 | 13.17 |
| Tyranton | 3.44 | 14.97 |
| 3–Octanol | 0.38 | 16.72 |
| Menthone | 4.46 | 18.68 |
| 1–Octen-3–ol | 1.06 | 18.90 |
| Isomenthone | 30.34 | 19.82 |
| Isopulegone | 0.49 | 22.90 |
| Caryophyllene | 1.38 | 23.68 |
| (R)-Pulegone | 40.75 | 25.73 |
| Borneol | 1.43 | 28.14 |
| cis-Jasmone | 0.11 | 36.03 |
3. Materials and Methods
3.1. Collection of the plant material
3.2. Isolation of the essential oil
3.3. GC-MS analysis
3.4. HPLC analysis
3.5. Minimum inhibitory concentration (MIC)
3.6. Fungicidal or fungistatic activity
3.7. Statistical analysis
4. Conclusions
Acknowledgment
References
- Blum, E. Molecular targets for prevention of hepatocellular carcinoma. Dig. Des. 2002, 20, 81–90. [Google Scholar] [CrossRef]
- Van Vleet, T.R.; Klein, P.J.; Coulombe, R.A. Metabolism and cytotoxicity of aflatoxin in cytochrome p-450 expressing human lung cells. J. Toxicol. Environ. Health 2002, 65, 853–886. [Google Scholar]
- Diop, Y.M.; Diuf, M.A.; Fall, A.; Thiam, B.; Ndiaye, M.; Ciss, D. Pesticide bioaccumulation: measurement and levels of organochloride residues in products of vegetables origin. Dakar Med. 1999, 44, 153–157. [Google Scholar]
- Lopez, P.; Sánchez, C.; Batlle, R.; Nerín, C. Solid- and vapor-phase antimicrobial activities of six essential oils: Susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef]
- Lopez, P.; Sánchez, C.; Batlle, R.; Nerín, C. Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J. Agric. Food Chem. 2007, 55, 4348–4356. [Google Scholar] [CrossRef]
- Helal, G.A.; Sarhan, M.M.; Abu Shahla, A.N.K.; El-Khair, E.K.A. Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strain. J. Basic Microbiol. 2007, 47, 5–15. [Google Scholar] [CrossRef]
- Ruiz Navajas, Y.; Viuda Martos, M.; Fernandez Lopez, J.; Perez Alvarez, J.A. Chemical composition and antifungal properties of essential oils from mandarin (Citrus reticulata L.) and grapefruit (Citrus paradise L.). Alimen. Equipos. Tecnol. 2006, 25, 81–85. [Google Scholar]
- Chimal, A.; González-Medrano, F.; Díaz, I.; Hernández, A.; Noriega, R.; Bravo, E.; Pérez, J.; Vázquez, J. Investigación sobre flora y fauna silvestres de la Reserva de la Biósfera "El Cielo", Tamaulipas; Secretaría de Desarrollo Urbano y Ecología (SEDUE) and Universidad Autónoma Metropolitana-Xochimilco: Mexico, D.F., Mexico, 1989; p. 106. [Google Scholar]
- Garcia, C.M.L. Estudio fitoquímico de Hesperozypis marifolia (Labiatae or Lamiaceae). Tesis de Licenciatura, Facultad de Química, UNAM, México D.F., Mexico, 1994. [Google Scholar]
- Von Poser, G.L.; Menut, C.; Toffoli, M.E.; Vérin, P.; Sobral, M.; Bessière, J.-M.; Lamaty, G.; Henriques, A.T. Essential Oil Compositioon and Allelopathic Effect of the Brazilian Lamiaceae Hesperozygis ringens (Benth.) Epling and Hesperozygis rhododon Epling. J. Agric. Food Chem. 1996, 44, 1829–1832. [Google Scholar]
- Cárdenas-Ortega, N.; Zavala-Sánchez, M.A.; Aguirre-Rivera, J.R.; Pérez-González, C.; Pérez-Gutiérrez, S. Chemical composition and antifungal activity of essential oil of Chrysactinia mexicana Gray. J. Agric. Food Chem. 2005, 53, 4347–4349. [Google Scholar] [CrossRef]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and antifungal activity of essential oil of Mentha suaveolens. Phytother. Res. 2002, 16, 727–731. [Google Scholar] [CrossRef]
- Ruth, N.; Kalck, P.; Roques, C.; Roux, I.; Michel, G. Comparison of antimicrobial properties of monoterpenes and their carbonylated products. Planta Med. 1996, 62, 275–277. [Google Scholar] [CrossRef]
- Flamini, G.; Gioni, P.L.; Puleio, R.; Morelli, L.; Panizzi, L. Antimicrobial activity of the essential oil of Calamintha nepeta and its constituent pulegone against bacteria and fungi. Phytother. Res. 1999, 13, 349–351. [Google Scholar] [CrossRef]
- Kim, J.S.; Seo, D.K.; Jang, S.A.; Han, J.H., Kim; Kim, G.H. Fumigant toxicity of 18 essential oils and their major compounds against adult longicorn beetle, Moechotypa diphysis (Coleoptera: Cerambycidae). J. Appl. Entomol. 2006, 45, 189–194. [Google Scholar]
- Sample Availability: Samples of the compounds and essential oil are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
González-Chávez, M.M.; Cárdenas-Ortega, N.C.; Méndez-Ramos, C.A.; Pérez-Gutiérrez, S. Fungicidal Properties of the Essential Oil of Hesperozygis marifolia on Aspergillus flavus Link. Molecules 2011, 16, 2501-2506. https://doi.org/10.3390/molecules16032501
González-Chávez MM, Cárdenas-Ortega NC, Méndez-Ramos CA, Pérez-Gutiérrez S. Fungicidal Properties of the Essential Oil of Hesperozygis marifolia on Aspergillus flavus Link. Molecules. 2011; 16(3):2501-2506. https://doi.org/10.3390/molecules16032501
Chicago/Turabian StyleGonzález-Chávez, Marco Martín, Norma Cecilia Cárdenas-Ortega, Cristina Andrea Méndez-Ramos, and Salud Pérez-Gutiérrez. 2011. "Fungicidal Properties of the Essential Oil of Hesperozygis marifolia on Aspergillus flavus Link" Molecules 16, no. 3: 2501-2506. https://doi.org/10.3390/molecules16032501