Salidroside Attenuates Hydrogen Peroxide-Induced Cell Damage Through a cAMP-Dependent Pathway
Abstract
:1. Introduction

2. Results and Discussion
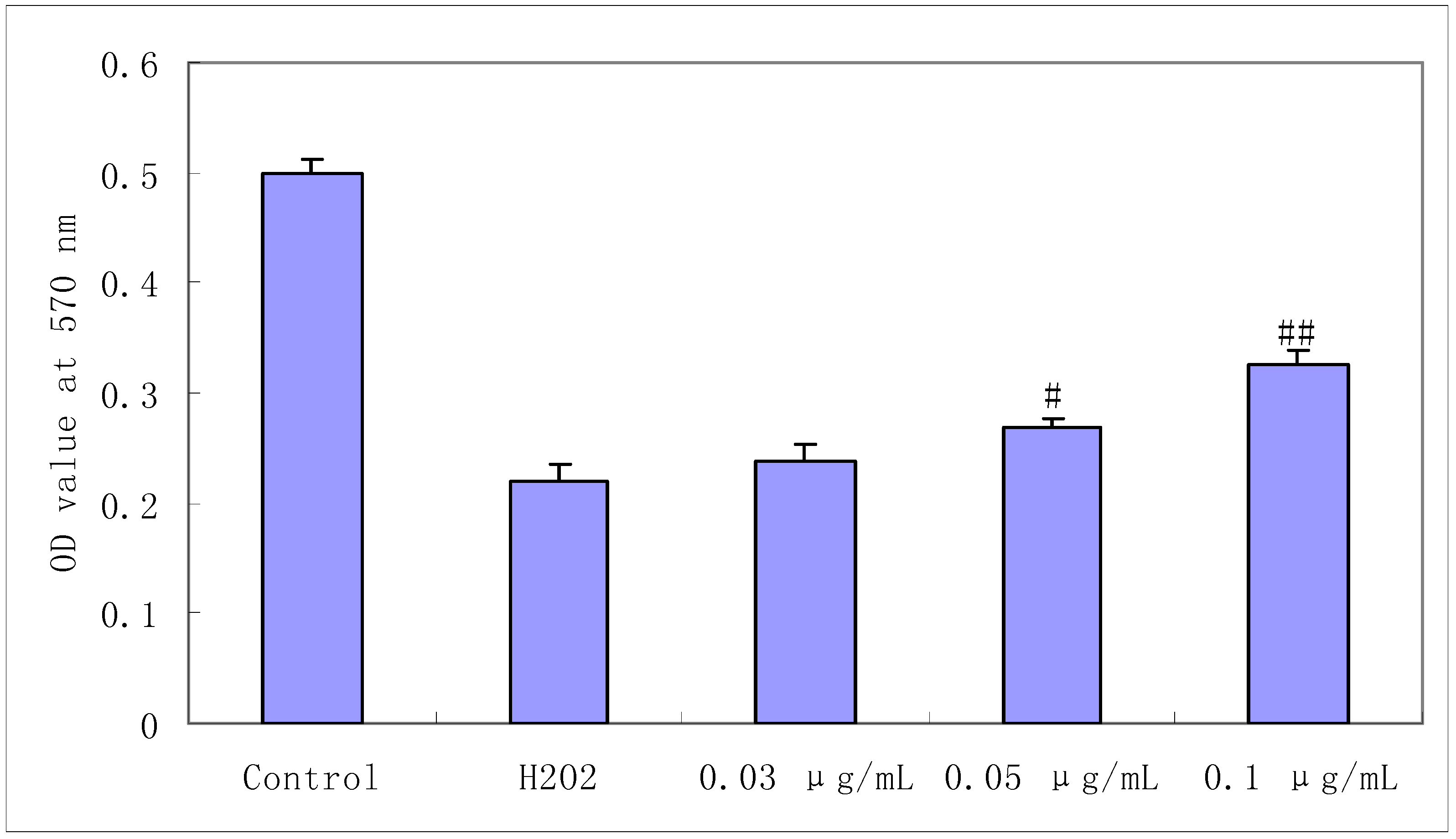
2.1. Protective Effect of Salidroside on H2O2-Induced Cell Damage
2.2. Salidroside Inhibited H2O2-Induced [Ca2+]i Elevation

2.3. Effects of Salidroside on the Formation of cAMP and cGMP

2.4. Salidroside Inhibited H2O2-Induced ROS Elevation

2.5. Discussion
3. Experimental
3.1. Materials
3.2. Protective Effect of Salidroside on H2O2-Induced Cell Damage
3.3. Determination of [Ca2+]i
3.4. Measurement of cAMP and cGMP
3.5. Measurement of ROS
3.6. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Ma, C.Y.; Tang, J.; Wang, H.X.; Tao, G.J.; Gu, X.H.; Hu, L. Preparative purification of salidroside from Rhodiola rosea by two-step adsorption chromatography on resins. J. Sep. Sci. 2009, 32, 185–191. [Google Scholar]
- Mao, G.X.; Deng, H.B.; Yuan, L.G.; Li, D.D.; Li, Y.Y.Y.; Wang, Z. Protective role of salidroside against aging in a mouse model induced by D-galactose. Biomed. Environ. Sci. 2010, 23, 161–166. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhang, X.Q.; Qiu, S.F.; Yu, D.H.; Lin, S.X. Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 2010, 398, 62–67. [Google Scholar] [CrossRef]
- Skopinska-Rozewska, E.; Malinowski, M.; Wasiutynski, A.; Sommer, E.; Furmanowa, M.; Mazurkiewicz, M.; Siwicki, A.K. The influence of Rhodiola quadrifida 50% hydro-alcoholic extract and salidroside on tumor-induced angiogenesis in mice. Pol. J. Vet. Sci. 2008, 11, 97–104. [Google Scholar]
- Li, M.H.; Zhang, G.Z.; Wang, Y.S. Effect of salidroside on salivary adenoid cystic carcinoma cells in vitro. Hua Xi Kou Qiang Yi Xue Za Zhi 2008, 36, 312–315, 319. [Google Scholar]
- Zhu, J.B.; Wan, X.Y.; Zhu, Y.P.; Ma, X.L.; Zheng, Y.W.; Zhang, T.B. Evaluation of salidroside in vitro and in vivo genotoxicity. Drug Chem. Toxicol. 2010, 33, 220–222. [Google Scholar] [CrossRef]
- Wang, H.B.; Ding, Y.Y.; Zhou, J.; Sun, X.L.; Wang, S.W. The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine 2009, 16, 146–155. [Google Scholar]
- Zuo, G.Y.; Li, Z.Q.; Chen, L.R.; Xu, X.J. Activity of compounds from Chinese herbal medicine Rhodiola kirilowii (Regel) Maxim against HCVNS3 serine protease. Antivir. Res. 2007, 76, 86–92. [Google Scholar] [CrossRef]
- Wu, Y.L.; Piao, D.M.; Han, X.H.; Nan, J.X. Protective effects of salidroside against acetaminophen-induced toxicity in mice. Biol. Pharm. Bull. 2008, 31, 1523–1529. [Google Scholar] [CrossRef]
- Zou, Y.Q.; Cai, Z.Y.; Mao, Y.F.; Li, J.B.; Deng, X.M. Effects of salidroside-pretreatment on neuroethology of rats after global cerebral ischemia-reperfusion. Zhong Xi Yi Jie He Xue Bao 2009, 7, 130–134. [Google Scholar] [CrossRef]
- Yu, S.; Liu, M.; Gu, X.; Ding, F. Neuroprotective effects of salidroside in the pc12 cell model exposed to hypoglycemia and serum limitation. Cell. Mol. Neurobiol. 2008, 28, 1067–1078. [Google Scholar]
- Liang, X.Q.; Xie, P.; Zhang, Y.; Shi, T.; Wang, Q.J.; Yan, T.H. Effects of salidroside on myocardial ischemia/reperfusion injury in rats. Chin. J. Nat. Med. 2010, 8, 127–131. [Google Scholar] [CrossRef]
- Wu, T.J.; Zhou, H.P.; Jin, Z.X.; Bi, S.H.; Yang, X.L.; Yi, D.H.; Liu, W.Y. Cardioprotection of salidroside from ischemia/reperfusion injury by increasing N-acetylglucosamine linkage to cellular proteins. Eur. J. Pharmacol. 2009, 613, 93–99. [Google Scholar]
- Li, H.B.; Ge, Y.K.; Zheng, X.X.; Zhang, L. Salidroside stimulated glucose uptake in skeletal muscle cells by activating AMP-activated protein kinase. Eur. J. Pharmacol. 2008, 588, 165–169. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Lazar, M.; Molino, B.; Rodriguez, R.; Davidov, T.; Su, J.; Tse, J.; Weiss, H.R.; Scholz, P.M. Reduction in interaction between cGMP and cAMP in dog ventricular myocytes with hypertrophic failure. Amer. J. Physiol.-Heart Circ. Phy. 2005, 289, H1251–H1257. [Google Scholar]
- Zhang, Li.; Yu, H.; Zhao, X.; Lin, X.; Tan, C.; Cao, G.; Wang, Z. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int. 2010, 57, 547–555. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, H.H.; Kim, S.; Lee, K.T.; Ham, I.H.; Whang, W.K. Antioxidant compounds from quercus salicina blume stem. Arch. Pharm. Res. 2008, 31, 274–278. [Google Scholar] [CrossRef]
- Monroe, R.K.; Halvorsen, S.W. Environmental toxicants inhibit neuronal Jak tyrosine kinase by mitochondrial disruption. Neurotoxicology 2009, 30, 589–598. [Google Scholar] [CrossRef]
- Cai, L.; Wang, H.; Li, Q.; Qian, Y.F.; Yao, W.B. Salidroside inhibits H2O2-induced apoptosis in PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Acta Biochim. Biophys. Sinica 2008, 40, 796–802. [Google Scholar]
- Csordas, G.; Hajnoczky, G. SR/ER-mitochondrial local communication: Calcium and ROS. Biochim. Biophys. Acta 1787, 1352–1362. [Google Scholar]
- Gerich, F.J.; Funke, F.; Hildebrandt, B.; Fasshauer, M.; Muller, M. H2O2-mediated modulation of cytosolic signaling and organelle function in rat hippocampus. Pflugers Arch.-Eur. J. Phys. 2009, 458, 937–952. [Google Scholar] [CrossRef]
- White, R.; Gibson, J.S. The effect of oxygen tension on calcium homeostasis in bovine articular chondrocytes. J. Orthop. Surg. Res. 2010, 5, 27. [Google Scholar] [CrossRef]
- Mironov, S.L.; Skorova, E.; Taschenberger, G.; Hartelt, N.; Nikolaev, V.O.; Lohse, M.J.; Kugler, S. Imaging cytoplasmic cAMP in mouse brainstem neurons. BMC Neurosci. 2009, 10, 29. [Google Scholar]
- Cho, H.J.; Cho, J.Y.; Rhee, M.H.; Park, H.J. Cordycepin (3’-deoxyadenosine) inhibits human platelet aggregation in a cyclic AMP- and cyclic GMP-dependent manner. Eur. J. Pharmacol. 2007, 558, 43–51. [Google Scholar] [CrossRef]
- Hemmrich, K.; Gummersbach, C.; Paul, N.E.; Goy, D.; Suschek, C.V.; Kroncke, K.D.; Pallua, N. Nitric oxide and downstream second messenger cGMP and cAMP enhance adipogenesis in primary human preadipocytes. Cytotherapy 2010, 12, 547–553. [Google Scholar] [CrossRef]
- Yamashiro, K.; Sasano, T.; Tojo, K.; Namekata, I.; Kurokawa, J.; Sawada, N.; Suganami, T.; Kamei, Y.; Tanaka, H.; Tajima, N.; et al. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem. Biophs. Res. Commun. 2010, 398, 284–289. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the salidroside are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guan, S.; Wang, W.; Lu, J.; Qian, W.; Huang, G.; Deng, X.; Wang, X. Salidroside Attenuates Hydrogen Peroxide-Induced Cell Damage Through a cAMP-Dependent Pathway. Molecules 2011, 16, 3371-3379. https://doi.org/10.3390/molecules16043371
Guan S, Wang W, Lu J, Qian W, Huang G, Deng X, Wang X. Salidroside Attenuates Hydrogen Peroxide-Induced Cell Damage Through a cAMP-Dependent Pathway. Molecules. 2011; 16(4):3371-3379. https://doi.org/10.3390/molecules16043371
Chicago/Turabian StyleGuan, Shuang, Wei Wang, Jing Lu, Wenhui Qian, Guoren Huang, Xuming Deng, and Xuelin Wang. 2011. "Salidroside Attenuates Hydrogen Peroxide-Induced Cell Damage Through a cAMP-Dependent Pathway" Molecules 16, no. 4: 3371-3379. https://doi.org/10.3390/molecules16043371
APA StyleGuan, S., Wang, W., Lu, J., Qian, W., Huang, G., Deng, X., & Wang, X. (2011). Salidroside Attenuates Hydrogen Peroxide-Induced Cell Damage Through a cAMP-Dependent Pathway. Molecules, 16(4), 3371-3379. https://doi.org/10.3390/molecules16043371



