Copper(I)-Catalyzed [ 3+ 2] Cycloaddition of 3-Azidoquinoline-2,4(1H,3H)-diones with Terminal Alkynes †
Abstract
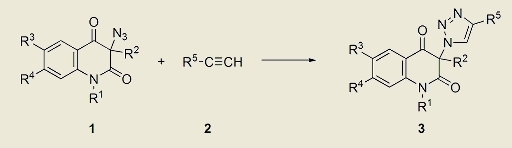
:1. Introduction

2. Results and Discussion

| Entry | Azide | Alkyne | Triazole | R. time, h | Yield, % b | |||||
| 1 | R1 | R2 | R3 | R4 | 2 | R5 | 3 | |||
| 1 | 1A | H | Ph | H | H | 2a | Ph | 3Aa | 3.3 | 96 (80) |
| 2 | 1A | H | Ph | H | H | 2b | CH2OH | 3Ab | 1.5 | 95 (67) |
| 3 | 1A | H | Ph | H | H | 2c | 3-(NH2)-C6H4 | 3Ac | 1.3 | nd (61) |
| 4 | 1B | H | Ph | MeO | H | 2a | Ph | 3Ba | 2.2 | 98 (64) |
| 5 | 1B | H | Ph | MeO | H | 2b | CH2OH | 3Bb | 1.1 | 85 (nd) |
| 6 | 1C | H | Pr | MeO | H | 2a | Ph | 3Ca | 1.2 | 97 (78) |
| 7 | 1C | H | Pr | MeO | H | 2b | CH2OH | 3Cb | 3 | nd (60) |
| 8 | 1D | H | Ph | Cl | MeO | 2a | Ph | 3Da | 1.2 | 92 (82) |
| 9 | 1D | H | Ph | Cl | MeO | 2b | CH2OH | 3Db | 3 | nd (46) |
| 10 | 1E | Bn | Ph | H | H | 2a | Ph | 3Ea | 2.5 | 97 (51) |
| 11 | 1E | Bn | Ph | H | H | 2b | CH2OH | 3Eb | 2 | 94 (89) |

3. Experimental
3.1. General

3.2. 4-Hydroxy-6-methoxy-3-propylquinolin-2(1H)-one (4C)
3.3. 3-Chloro-6-methoxy-3-propylquinoline-2,4(1H,3H)-dione (5C)
3.4. Chlorination of 6-chloro-4-hydroxy-7-methoxy-3-phenylquinolin-2(1H)-one (4D) into 3,6-Dichloro-7-methoxy-3-phenylquinoline-2,4(1H,3H)-dione (5D) and 3,6,8-Trichloro-7-methoxy-3-phenylquinoline-2,4(1H,3H)-dione (5F)
3.5. General Procedure for the Synthesis 3-Azidoquinoline-2,4(1H,3H)-diones 1A–D
3.6. General Procedure for the Preparation of 1,2,3-Triazoles 3
3.7. One-Pot Synthesis of 3-Phenyl-3-(4-phenyl-1H-1,2,3-triazol-1-yl)quinoline-2,4(1H,3H)-dione (3Aa) Starting from 3-Bromo-3-phenylquinoline-2,4(1H,3H)-dione (6A)
3.8. N-(3-(1-(1,2,3,4-Tetrahydro-2,4-dioxo-3-phenylquinolin-3-yl)-1H-1,2,3-triazol-4-yl)phenyl)acetamide (3Ae).
4. Conclusions
Acknowledgements
References and Notes
- Kafka, S.; Klásek, A.; Polis, J.; Košmrlj, J. Syntheses of 3-aminoquinoline-2,4(1H,3H)-diones. Heterocycles 2002, 57, 1659–1682. [Google Scholar] [CrossRef]
- Gololobov, Y.G.; Kasukhin, L.F. Recent advances in the Staudinger reaction. Tetrahedron 1992, 48, 1353–1406. [Google Scholar] [CrossRef]
- L’abbé, G. Are azidocumulenes accessible? Bull. Soc. Chim. Belg. 1984, 93, 579–592. [Google Scholar]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006, 2006, 51–68. [Google Scholar]
- Appukkuttan, P.; Van der Eycken, E. Recent developments in microwave-assisted, transition-metal-catalysed C–C and C–N bond-forming reactions. Eur. J. Org. Chem. 2008, 2008, 1133–1155. [Google Scholar] [CrossRef]
- Kappe, C.O.; Van der Eycken, E. Click chemistry under non-classical reaction conditions. Chem. Soc. Rev. 2010, 39, 1280–1290. [Google Scholar] [CrossRef]
- Urankar, D.; Košmrlj, J. Concise and diversity-oriented synthesis of ligand arm-functionalized azoamides. J. Comb. Chem. 2008, 10, 981–985. [Google Scholar] [CrossRef]
- Urankar, D.; Košmrlj, J. Preparation of diazenecarboxamide-carboplatin conjugates by click chemistry. Inorg. Chim. Acta 2010, 363, 3817–3822. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Multicomponent synthesis of 1,2,3-triazoles in water catalyzed by copper nanoparticles on activated carbon. Adv. Synth. Catal. 2010, 352, 3208–3214. [Google Scholar] [CrossRef]
- Kumar, D.; Patel, G.; Reddy, V.B. Greener and expeditious synthesis of 1,4-disubstituted 1,2,3-triazoles from terminal acetylenes and in situ generated alpha-azido ketones. Synlett 2009, 3, 399–402. [Google Scholar]
- Kashinath, D.; Budin, G.; Baati, R.; Meunier, S.; Wagner, A. Azidation of β-carbonyl lactones and lactams. Tetrahedron Lett. 2009, 50, 5379–5381. [Google Scholar]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides: An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef]
- Bräse, S.; Banert, K. Organic Azides: Syntheses and Applications; John Wiley & Sons: Chichester, UK, 2010. [Google Scholar]
- Stadlbauer, W.; Schmut, O.; Kappe, T. Synthesis of benzofurans by cyclodehydrogenation of phenylmalonyl heterocyclics. Monatsh. Chem. 1980, 111, 1005–1013. [Google Scholar]
- Stadlbauer, W.; Kappe, T. Synthesis of benzofurans by cyclodehydrogenation of phenylmalonyl heterocyclics. Monatsh. Chem. 1985, 116, 1005–1015. [Google Scholar] [CrossRef]
- Stadlbauer, W.; Laschober, R.; Lutschounig, H.; Schindler, G.; Kappe, T. Organic azides in heterocycle synthesis. Part 16. Halogenation reactions in position 3 of quinoline-2,4-dione systems by electrophilic substitution and halogen exchange. Monatsh. Chem. 1992, 123, 617–636. [Google Scholar]
- Sample Availability: Samples of the compounds 1–6 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kafka, S.; Hauke, S.; Salcinovic, A.; Soidinsalo, O.; Urankar, D.; Kosmrlj, J. Copper(I)-Catalyzed [ 3+ 2] Cycloaddition of 3-Azidoquinoline-2,4(1H,3H)-diones with Terminal Alkynes. Molecules 2011, 16, 4070-4081. https://doi.org/10.3390/molecules16054070
Kafka S, Hauke S, Salcinovic A, Soidinsalo O, Urankar D, Kosmrlj J. Copper(I)-Catalyzed [ 3+ 2] Cycloaddition of 3-Azidoquinoline-2,4(1H,3H)-diones with Terminal Alkynes. Molecules. 2011; 16(5):4070-4081. https://doi.org/10.3390/molecules16054070
Chicago/Turabian StyleKafka, Stanislav, Sylvia Hauke, Arjana Salcinovic, Otto Soidinsalo, Damijana Urankar, and Janez Kosmrlj. 2011. "Copper(I)-Catalyzed [ 3+ 2] Cycloaddition of 3-Azidoquinoline-2,4(1H,3H)-diones with Terminal Alkynes" Molecules 16, no. 5: 4070-4081. https://doi.org/10.3390/molecules16054070