The Effect of Ultrasound on the Functional Properties of Wheat Gluten
Abstract
:1. Introduction
2. Results and Discussion
2.1. Temperature Changes in Ultrasound Treated Wheat Gluten Suspensions

2.2. Foaming Properties of Ultrasound Treated Wheat Gluten


2.3. Emulsifying Properties of Ultrasound Treated Wheat Gluten
2.4. SDS-PAGE Patterns of Wheat Gluten Proteins
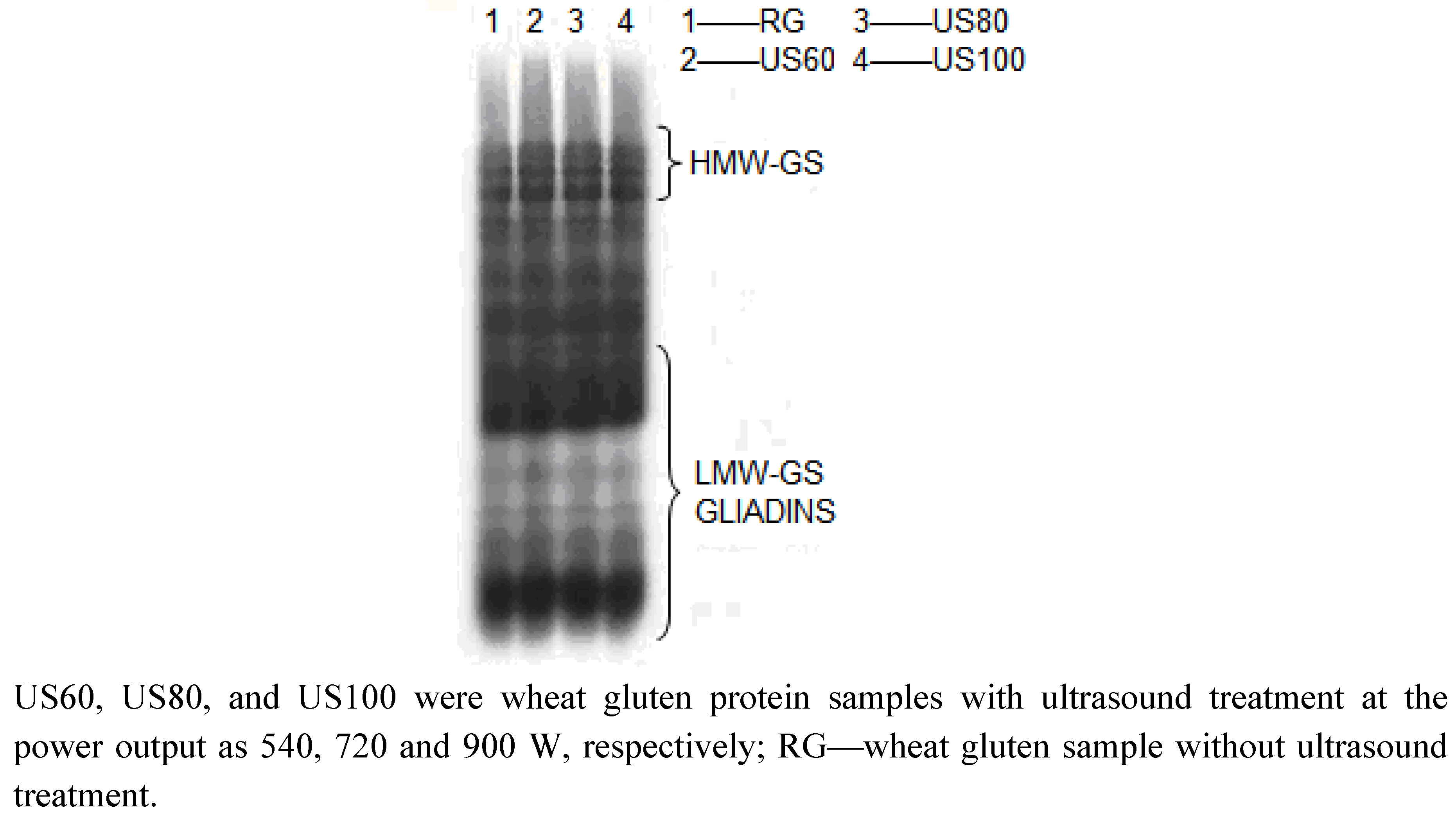
2.5. Dynamic Rheological Properties of Ultrasound Treated Wheat Gluten

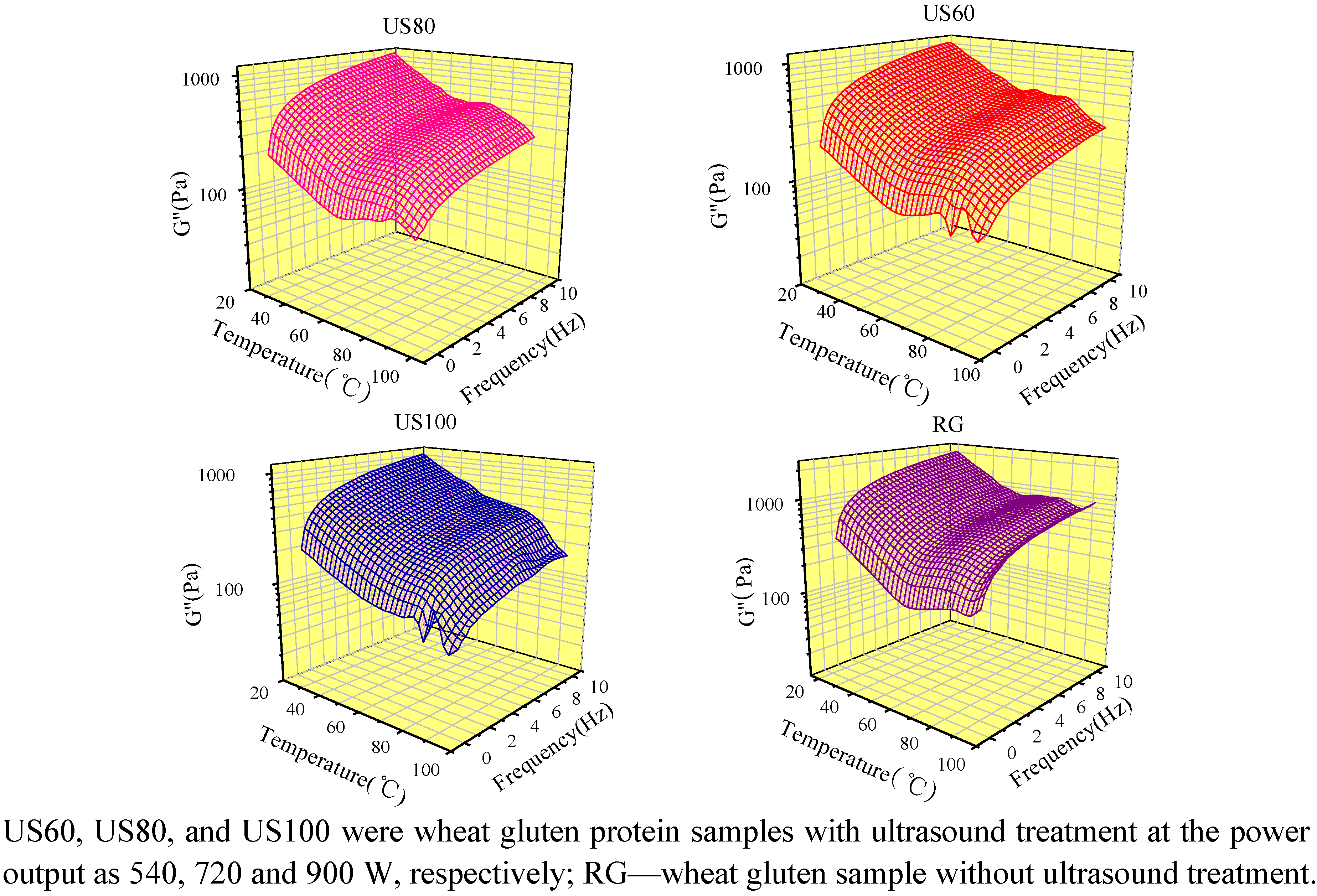
3. Experimental
3.1. Materials and Chemicals
3.2. Ultrasound Treatment Protocol
3.3. Foaming Capacity and Foam Stability
3.4. Emulsifying Properties


3.5. SDS-PAGE Analysis
3.6. Dynamic Rheological Measurements
3.7. Statistic Analysis
4. Conclusions
Acknowledgements
References
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Tech. 2010, 21, 323–331. [Google Scholar]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Tech. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Barbosa-Ca'novas, G.V.; Rodrı'guez, J.J. Update on nonthermal food processing technologies: Pulsed electric field, high hydrostatic pressure, irradiation and ultrasoun. Food Aust. 2002, 54, 513–518. [Google Scholar]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- DemirdÖven, A.; Baysal, T. The use of ultrasound and combined technologies in food preservation. Food Rev. Int. 2009, 25, 1–11. [Google Scholar] [CrossRef]
- Gülseren, I.; Güzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183. [Google Scholar] [CrossRef]
- Toufeili, I.; Ismail, B.; Shadarevian, S.; Baalbaki, R.; Khatkar, B.S.; Bell, A.E.; Schofield, J.D. The role of gluten proteins in the baking of arabic bread. J. Cereal Sci. 1999, 30, 255–265. [Google Scholar] [CrossRef]
- Kayserilioğlu, B.Ş.; Bakir, U.; Yilmaz, L.; Akkaş, N. Drying temperature and relative humidity effects on wheat gluten film properties. J. Agr. Food Chem. 2003, 51, 964–968. [Google Scholar]
- Larre, C.; Desserme, C.; Barbot, J.; Gueguen, J. Properties of deamidated gluten films enzymatically cross-linked. J. Agr. Food Chem. 2000, 48, 5444–5449. [Google Scholar] [CrossRef]
- Singh, H.; MacRitchie, F. Changes in proteins induced by heating gluten dispersions at high temperature. J. Cereal Sci. 2004, 39, 297–301. [Google Scholar] [CrossRef]
- Stathopoulos, C.E.; Tsiami, A.A.; Schofield, J.D.; Dobraszczyk, B.J. Effect of heat on rheology, surface hydrophobicity and molecular weight distribution of glutens extracted from flours with different bread-making quality. J. Cereal Sci. 2008, 47, 134–143. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; van der Goot, A.J.; Hamer, R.J.; Boom, R.M. Effect of simple shear on the physical properties of glutenin macro polymer (GMP). J. Cereal Sci. 2005, 42, 59–68. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, M.M.; Ren, J.Y.; Zhao, H.F.; Cui, C.; Hu, X. Effect of acetic acid deamidation-induced modification on functional and nutritional properties and conformation of wheat gluten. J. Sci. Food Agr. 2010, 90, 409–417. [Google Scholar]
- Drago, S.R.; Gonzalez, R.J. Foaming properties of enzymatically hydrolysed wheat gluten. Innov. Sci. Emerg. Technol. 2001, 1, 269–273. [Google Scholar]
- Wang, J.S.; Zhao, M.M.; Yang, X.Q.; Jiang, Y.M. Improvement on functional properties of wheat gluten by enzymatic hydrolysis and ultrafiltration. J. Cereal Sci. 2006, 44, 93–100. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Lelas, V.; Mason, T.J.; Krešic, G.; Badanjak, M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009, 93, 386–393. [Google Scholar] [CrossRef]
- Kresic, G.; Lelas, V.; Jambrak, A.R.; Herceg, Z.; Brncic, S.R. Influence of novel food processing technologies on the rheological and thermophysical properties of whey proteins. J. Food Eng. 2008, 87, 64–73. [Google Scholar] [CrossRef]
- Lim, K.S.; Barigou, M. Ultrasound-Assisted Generation of Foam. Ind. Eng. Chem. Res. 2005, 44, 3312–3320. [Google Scholar]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Pongsawatmanit, R.; Harnsilawat, T.; McClements, D.J. Influence of alginate, pH and ultrasound treatment on palm oil-in-water emulsions stabilized by b-lactoglobulin. Colloids Surfaces A 2006, 287, 59–67. [Google Scholar]
- Seshadri, R.; Weiss, J.; Hulbert, G.J.; Mount, J. Ultrasonic processing influences rheological and optical properties of high-methoxyl pectin dispersions. Food Hydrocolloids 2003, 17, 191–197. [Google Scholar]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- He, H.; Hoseney, R.C. Gas retention in bread dough during baking. Cereal Chem. 1991, 68, 521–525. [Google Scholar]
- Tian, Z.M.; Wan, M.X.; Wang, S.P.; Kang, J.Q. Effects of ultrasound and additives on the function and structure of trypsin. Ultrason. Sonochem. 2004, 11, 399–404. [Google Scholar]
- Sample Availability: Contact the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, H.; Claver, I.P.; Zhu, K.-X.; Zhou, H. The Effect of Ultrasound on the Functional Properties of Wheat Gluten. Molecules 2011, 16, 4231-4240. https://doi.org/10.3390/molecules16054231
Zhang H, Claver IP, Zhu K-X, Zhou H. The Effect of Ultrasound on the Functional Properties of Wheat Gluten. Molecules. 2011; 16(5):4231-4240. https://doi.org/10.3390/molecules16054231
Chicago/Turabian StyleZhang, Haihua, Irakoze P. Claver, Ke-Xue Zhu, and Huiming Zhou. 2011. "The Effect of Ultrasound on the Functional Properties of Wheat Gluten" Molecules 16, no. 5: 4231-4240. https://doi.org/10.3390/molecules16054231
APA StyleZhang, H., Claver, I. P., Zhu, K. -X., & Zhou, H. (2011). The Effect of Ultrasound on the Functional Properties of Wheat Gluten. Molecules, 16(5), 4231-4240. https://doi.org/10.3390/molecules16054231



