Monasnicotinates A–D, Four New Pyridine Alkaloids from the Fungal Strain Monascus pilosus BCRC 38093
Abstract
:1. Introduction

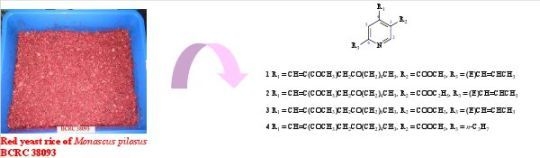
2. Results and Discussion
| No. | 1 | 2 | |||
| δH | δC | δH | δC | ||
| 2 | 9.13 (s) | 151.8 | 9.14 (s) | 151.7 | |
| 3 | 121.1 | 121.4 | |||
| 4 | 145.7 | 145.7 | |||
| 5 | 7.33 (s) | 120.1 | 7.24 (s) | 120.1 | |
| 6 | 159.4 | 159.2 | |||
| 7 | 6.54 (dd, J = 16.0, 1.8) | 130.2 | 6.54 (dd, J = 16.0, 2.0) | 130.1 | |
| 8 | 6.93 (dq, J = 16.0, 7.0) | 135.4 | 6.92 (dq, J = 16.0, 7.0) | 135.5 | |
| 9 | 1.96 (dd, J = 7.0, 1.8) | 18.6 | 1.95 (dd, J = 7.0, 2.0) | 18.7 | |
| 10 | 165.5 | 165.1 | |||
| MeO-12 | 3.92 (s) | 52.3 | |||
| 12 | 4.36 (q, J = 7.2) | 61.4 | |||
| 13 | 8.13 (s) | 140.9 | |||
| Me-13 | 1.39 (t, J = 7.2) | 14.2 | |||
| C-14 | 136.9 | 141.0 | |||
| C-15 | 198.8 | 136.9 | |||
| Me-15 | 2.51 (s) | 25.4 | |||
| Me-16 | 2.50 (s) | 25.4 | |||
| 16 | 3.27 (s) | 40.6 | 198.8 | ||
| 17 | 208.7 | 3.27 (s) | 40.7 | ||
| 18 | 2.54 (d, J = 7.4) | 43.2 | 208.7 | ||
| 19 | 1.59 (m) | 23.4 | 2.54 (t, J = 6.8) | 43.2 | |
| 20 | 1.32 (m) | 31.3 | 1.59 (m) | 23.5 | |
| 21 | 1.27 (m) | 22.4 | 1.32 (m) | 31.3 | |
| Me-22 | 0.88 (t, J = 6.8) | 13.9 | |||
| 22 | 1.32 (m) | 22.4 | |||
| Me-23 | – | 0.87 (t, J = 7.2) | 13.9 | ||


| 3 | 4 | ||||
|---|---|---|---|---|---|
| δH | δC | δH | δC | ||
| 2 | 9.14 (s) | 151.8 | 9.16 (s) | 150.9 | |
| 3 | 121.1 | 121.5 | |||
| 4 | 145.8 | 146.2 | |||
| 5 | 7.24 (s) | 120.2 | 7.23 (s) | 122.8 | |
| 6 | 159.4 | 166.4 | |||
| 7 | 6.54 (dq, J = 15.6, 2.0) | 130.2 | 2.83 (t, J = 7.2) | 39.8 | |
| 8 | 6.92 (dq, J = 15.6, 7.0) | 135.6 | 1.75 (sext, J = 7.2) | 22.7 | |
| 9 | 1.95 (dd, J = 7.0, 2.0) | 18.7 | 0.94 (t, J = 7.2) | 13.7 | |
| 10 | 165.6 | 165.3 | |||
| MeO-12 | 3.92 (s) | 52.4 | 3.93 (s) | 52.5 | |
| 13 | 8.12 (s) | 140.9 | 8.13 (s) | 140.5 | |
| 14 | – | 137.0 | 137.2 | ||
| 15 | – | 198.8 | – | 198.7 | |
| Me-15 | 2.51 (s) | 25.4 | 2.51 (s) | 25.4 | |
| 16 | 3.27 (s) | 40.7 | 3.26 (s) | 40.7 | |
| 17 | – | 208.7 | 208.4 | ||
| 18 | 2.53 (d, J = 7.2) | 43.8 | 2.52 (t, J = 7.2) | 43.2 | |
| 19 | 1.58 (m) | 23.8 | 1.57 (m) | 23.4 | |
| 20 | 1.26 (m) | 31.6 | 1.26 (m) | 31.3 | |
| 21 | 1.26 (m) | 22.6 | 1.26 (m) | 22.4 | |
| 22 | 1.26 (m) | 29.1 | 0.88 (t, J = 7.2) | 13.9 | |
| 23 | 1.26 (m) | 22.6 | – | – | |
| 24 | 0.87 (t, J = 7.2) | 14.1 | – | – |
3. Experimental
3.1. General
3.2. Fungal Material
3.3. Fungal Material
3.4. Extraction and ISOLATION
3.5. Biological Assay
3.6. Spectral Data
4. Conclusions
Acknowledgements
References
- Akihisa, T.; Tokuda, H.; Yasukawa, K.; Ukiya, M.; Kiyota, A.; Sakamoto, N.; Suzuki, T.; Tanabe, N.; Nishino, H. Azaphilones, furanoisophthalides, and amino acids from the extracts of Monascus pilosus-fermented rice (red-mold rice) and their chemopreventive effects. J. Agric. Food Chem. 2005, 53, 562–565. [Google Scholar] [CrossRef]
- Endo, A.; Monacolin, K. A new hypocholesterolemic agent produced by Monascus species. J. Antibiot. 1979, 32, 852–854. [Google Scholar] [CrossRef]
- Kohama, Y.; Matsumoto, S.; Mimura, T.; Tanabe, N.; Inada, A.; Nakanishi, T. Isolation and identification of hypotensive principles in red-mold rice. Chem. Pharm. Bull. 1987, 35, 2484–2489. [Google Scholar] [CrossRef]
- Aniya, Y.; Ohtani, I.I.; Higa, T.; Miyagi, C.; Gibo, H.; Shimabukuro, M.; Nakanish, H.; Taira, J. Dimerumic acid as an antioxidantof the mold, Monascus anka. Free Radical Biol. Med. 1999, 286, 999–1004. [Google Scholar]
- Blanc, P.J.; Loret, M.O.; Goma, G. Production of citrinin by various species of Monascus. Biotechnol. Lett. 1995, 17, 291–294. [Google Scholar] [CrossRef]
- Tsuji, K.; Ichikawa, T.; Tanabe, N.; Abe, S.; Tarui, S.; Nakagawa, Y. Effects of two kinds of Koji on blood pressure in spontaneously hypertensive rats. Nippon. Nogeikagaku Kaishi. 1992, 66, 1241–1246. [Google Scholar]
- Endo, A. Compactin (ML-236B) and related compounds as potential cholesterol-lowering agents that inhibit HMG-CoA reductase. J. Med. Chem. 1985, 28, 401–405. [Google Scholar] [CrossRef]
- Martinokova, L.; Juzlova, P.; Vesely, D. Biological activity of polyketide pigments produced by the fungus Monascus. J. Appl. Bacteriol. 1995, 79, 609–616. [Google Scholar] [CrossRef]
- Wong, H.C.; Bau, Y.S. Pigmentation and antibiobacterial activity of fast neutron-and X-ray induced strains of Monascus purpureus went. Plant Physiol. 1977, 60, 578–581. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar]
- Akihisa, T.; Mafune, S.; Ukiya, M.; Kimura, Y.; Yasukawa, K.; Suzuki, T.; Tokuda, H.; Tanabe, N.; Fukuoka, T. (+)- and (–)-syn-2-Isobutyl-4-methylazetidine-2,4-dicarboxylic acids from the extract of Monascus pilosus-fermented rice (red-mold rice). J. Nat. Prod. 2004, 67, 479–480. [Google Scholar] [CrossRef]
- Cheng, M.J.; Wu, M.D.; Chen, I.S.; Yuan, G.F. Secondary metabolites from the mycelia of the fungus Monascus pilosus BCRC 38072. Chem. Pharm. Bull. 2008, 56, 394–397. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Y.; Li, L.; Li, Y. Two new Monascus metabolites with strong blue fluorescence isolated from red yeast rice. J. Agric. Food Chem. 2008, 56, 112–118. [Google Scholar] [CrossRef]
- Jongrungruangchok, S.; Kittakoop, P.; Yongsmith, B.; Bavovada, R.; Tanasupawat, S.; Lartpornmatulee, N.; Thebtaranonth, Y. Azaphilone pigments from a yellow mutant of the fungus Monascus kaoliang. Phytochemistry 2004, 65, 2569–2575. [Google Scholar] [CrossRef]
- Juzlová, P.; Martínková, L.; Kren, V. Secondary metabolites of the fungus Monascus: A review. J. Industrial Microbiol. 1996, 16, 163–170. [Google Scholar] [CrossRef]
- Juzlová, P.; Rezanka, T.; Martínková, L.; Kren, V. Long-chain fatty acids from Monascus purpureus. Phytochemistry 1996, 43, 151–153. [Google Scholar]
- Sato, K.; Goda, Y.; Sakamoto, S.S.; Shibata, H.; Maitani, T.; Yamada, T. Identification of major pigments containing D-amino acid units in commercial Monascus pigments. Chem. Pharm. Bull. 1997, 45, 227–229. [Google Scholar] [CrossRef]
- Wild, D.; Tóth, G.; Humpf, H.U. New Monascus metabolite isolated from red yeast rice (Angkak, red Koji). J. Agric. Food Chem. 2002, 50, 3999–4002. [Google Scholar] [CrossRef]
- Wild, D.; Tóth, G.; Humpf, H.U. New Monascus metabolites with a pyridine structure in red fermented rice. J. Agric. Food Chem. 2003, 51, 5493–5496. [Google Scholar] [CrossRef]
- Mosman, T. Utilization of purified human monocytes in the agarose droplet assay for measuring migration inhibitory factors. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Johansson, M.; Kopcke, B.; Anke, H.; Sterner, O. Biologically active secondary metabolites from the ascomycete A111-95. J. Antibiot. 2002, 55, 104–106. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1-4 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, M.-D.; Cheng, M.-J.; Yech, Y.-J.; Chen, Y.-L.; Chen, K.-P.; Chen, I.-S.; Yang, P.-H.; Yuan, G.-F. Monasnicotinates A–D, Four New Pyridine Alkaloids from the Fungal Strain Monascus pilosus BCRC 38093. Molecules 2011, 16, 4719-4727. https://doi.org/10.3390/molecules16064719
Wu M-D, Cheng M-J, Yech Y-J, Chen Y-L, Chen K-P, Chen I-S, Yang P-H, Yuan G-F. Monasnicotinates A–D, Four New Pyridine Alkaloids from the Fungal Strain Monascus pilosus BCRC 38093. Molecules. 2011; 16(6):4719-4727. https://doi.org/10.3390/molecules16064719
Chicago/Turabian StyleWu, Ming-Der, Ming-Jen Cheng, Yi-Jen Yech, Yen-Lin Chen, Kai-Ping Chen, Ih-Sheng Chen, Ping-Hsun Yang, and Gwo-Fang Yuan. 2011. "Monasnicotinates A–D, Four New Pyridine Alkaloids from the Fungal Strain Monascus pilosus BCRC 38093" Molecules 16, no. 6: 4719-4727. https://doi.org/10.3390/molecules16064719