Treatment of Alcohols with Tosyl Chloride Does Not always Lead to the Formation of Tosylates
Abstract
:1. Introduction

2. Results and Discussion
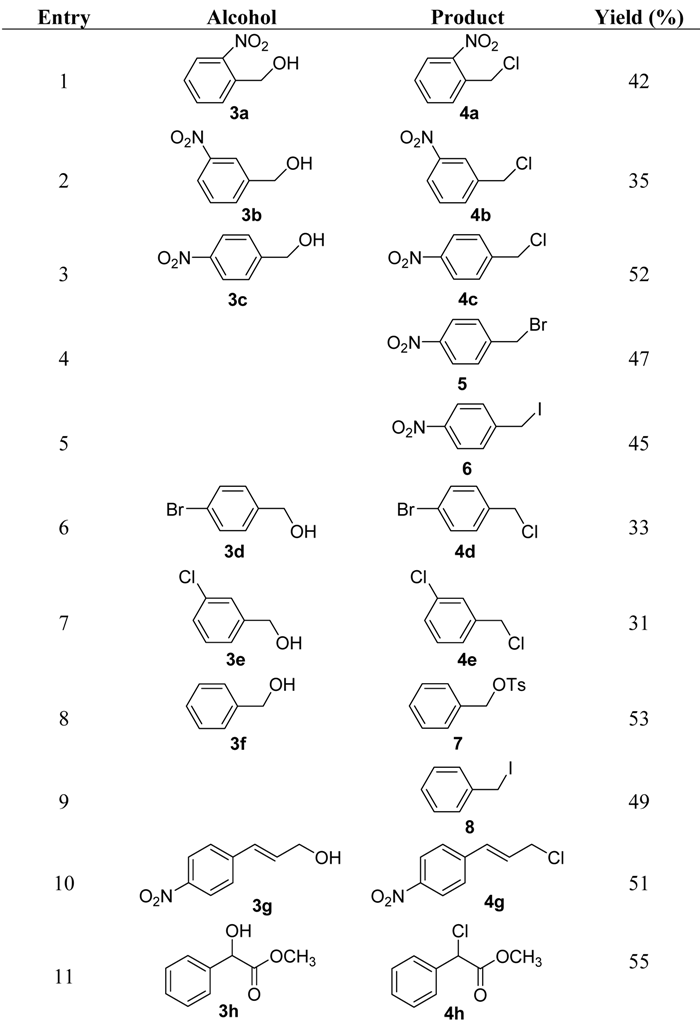 |

 |

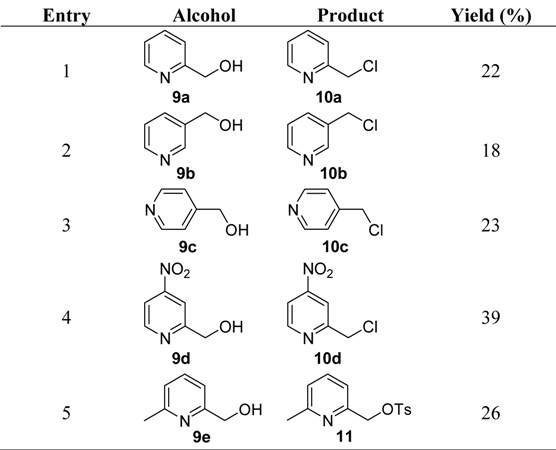 |
3. Experimental
3.1. General
3.2. General Procedure for Treatment of Alcohol with Tosyl Chloride
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References
- Vollhardt, K.P.C.; Schore, N.E. Organic Chemistry: Structure and Function, 3rd ed; W.H. Freeman: New York, NY, USA, 1999; pp. 342–343. [Google Scholar]
- Kabalka, G.W.; Varma, M.; Varma, R.S.; Srivastava, P.C.; Knapp, F.F., Jr. The tosylation of alcohols. J. Org. Chem. 1986, 51, 2386–2388. [Google Scholar] [CrossRef]
- Adlington, R.M.; Barrett, A.G.M.; Quayle, P.; Walker, A.; Betts, M.J. Novel syntheses of 3-methyleneazetidin-2-one derivatives and related systems. Chem. Commun. 1981, 404–405. [Google Scholar]
- Holand, S.; Epsztein, R. Preparation of oxiranes from vic-diols. Synthesis 1977, 706–708. [Google Scholar] [CrossRef]
- Albright, J.D.; Benz, E.; Lanziolotti, A.E.; Goldman, L. Reactions of sulfonyl chloride-N,N-dimethylformamide complexes. Chem. Commun. (London) 1965, 413–414. [Google Scholar]
- Smith, A.B.; Wan, Z. Total synthesis of the ansamycin antibiotic (+)-thiazinotrienomycin E. J. Org. Chem. 2000, 65, 3738–3753. [Google Scholar] [CrossRef]
- Yasuda, M.; Yamasaki, S.; Onishi, Y.; Baba, A. Indium-catalyzed direct chlorination of alcohols using chlorodimethylsilane-benzil as a selective and mild system. J. Am. Chem. Soc. 2004, 126, 7186–7187. [Google Scholar]
- Isobe, T.; Ishikawa, T. 2-Chloro-1,3-dimethylimidazolinium chloride. 3. Utility for chlorination, oxidation, reduction, and rearrangement reactions. J. Org. Chem. 1999, 64, 5832–5835. [Google Scholar] [CrossRef]
- Sun, L.; Peng, G.; Niu, H.; Wang, Q.; Li, C. A highly chemoselective and rapid chlorination of benzyl alcohols under neutral conditions. Synthesis 2008, 3919–3924. [Google Scholar]
- Sample Availability: Samples of the compounds mentioned above are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ding, R.; He, Y.; Wang, X.; Xu, J.; Chen, Y.; Feng, M.; Qi, C. Treatment of Alcohols with Tosyl Chloride Does Not always Lead to the Formation of Tosylates. Molecules 2011, 16, 5665-5673. https://doi.org/10.3390/molecules16075665
Ding R, He Y, Wang X, Xu J, Chen Y, Feng M, Qi C. Treatment of Alcohols with Tosyl Chloride Does Not always Lead to the Formation of Tosylates. Molecules. 2011; 16(7):5665-5673. https://doi.org/10.3390/molecules16075665
Chicago/Turabian StyleDing, Rui, Yong He, Xiao Wang, Jingli Xu, Yurong Chen, Man Feng, and Chuanmin Qi. 2011. "Treatment of Alcohols with Tosyl Chloride Does Not always Lead to the Formation of Tosylates" Molecules 16, no. 7: 5665-5673. https://doi.org/10.3390/molecules16075665




