New 3′,8′′-Linked Biflavonoids from Selaginella uncinata Displaying Protective Effect against Anoxia
Abstract
:1. Introduction
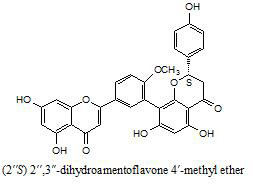
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation
3.4. The anoxic PC12 cells assay
4. Conclusions
Acknowledgements
References
- Jiangsu New Medical College. Dictionary of Chinese Herb Medicines; Shanghai Scientific and Technologic Press: Shanghai, China, 1986; pp. 1472–1473. [Google Scholar]
- Ma, L.Y.; Ma, S.C.; Wei, F.; Lin, R.C.; But, P.P.-H.; Lee, S.H.-S.; Lee, S.F. Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. 2003, 51, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.X.; Wang, N.L.; Gao, H.; Liu, H.W.; Chen, H.F.; Fan, M.; Yao, X.S. A new flavonoid with a benzoic acid substituent from Selaginella uncinata. Chin. Chem. Lett. 2008, 19, 1093–1095. [Google Scholar] [CrossRef]
- Zheng, J.X.; Wang, N.L.; Cheng, H.F.; Yao, X.S. Isolation and identification of phenolic constituents from Selaginella uncinata (Desv.) Spring. Zhongguo Yaowu Huaxue Zazhi 2007, 17, 302–305. [Google Scholar]
- Alvarez-Tejado, M.; Naranjo-Suárez, S.; Jiménez, C.; Carrera, A.C.; Landázuri, M.O.; Peso, L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J. Biol. Chem. 2001, 276, 22368–22374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.X.; Wang, N.L.; Liu, H.W.; Chen, H.F.; Li, M.M.; Wu, L.Y.; Fan, M.; Yao, X.S. Four new biflavonoids from Selaginella uncinata and their anti-anoxic effect. J. Asian Nat. Prod. Res. 2008, 10, 945–952. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Timmermann, B.N.; Aladesanmi, A.J.; Zeng, L. A biflavonoid from Dysoxylum lenticellare. Phytochemistry 1996, 42, 1199–1201. [Google Scholar] [CrossRef]
- Markham, K.R.; Sheppard, C.; Geiger, H. Carbon-13 NMR of flavonoids. Part IV. Carbon-13 NMR studies of some naturally occurring amentoflavone and hinokiflavone biflavonoids. Phytochemistry 1987, 26, 3335–3337. [Google Scholar]
- Ohmoto, T.; Yosida, O. Constituents of pollen. XI. Constituents of Cryptomeria japonica D. Don. Chem. Pharmaceut. Bull. 1983, 31, 919–924. [Google Scholar] [CrossRef]
- Swamy, R.C.; Kunert, O.; Schuhly, W. Structurally unique biflavonoids from Selaginella chrysocaulos and Selaginella bryopteris. Chem. Biodivers. 2006, 3, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.S.; Wang, N.L.; Fan, M.; Zheng, J.X.; Wu, L.Y.; Liu, H.W.; Ding, A.S.; Gao, H.; Dai, Y. Application of flavone derivatives as antioxidation and anti-hypoxia drug or food and their preparation. Chinese Patent CN101361733, 2009. [Google Scholar]
- Ahmed, I.; Ishratullah, K.; Ilyas, M.; Rahman, W.; Seligmann, O.; Wagner, H. Tetrahydroamentoflavone from nuts of Semecarpus prainii. Phytochemistry 1981, 20, 1169–1170. [Google Scholar] [CrossRef]
- Skopp, G.; Schwenker, G. Biflavonoids from Schinus terebinthifolius Raddi (Anacardiaceae). Z. Naturforsch. B 1986, 41B, 1479–1482. [Google Scholar] [CrossRef]
- Silva, G.L.; Chai, H.; Gupta, M.P.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Beecher, C.W.W.; Kinghorn, A. Cytotoxic biflavonoids from Selaginella willdenowii. Phytochemistry 1995, 40, 129–134. [Google Scholar] [CrossRef]
- Li, C.T.; Zhang, W.P.; Fang, S.H.; Lu, Y.B.; Zhang, L.H.; Qi, L.L.; Huang, X.Q.; Huang, X.J.; Wei, E.Q. Baicalin attenuates oxygen-glucose deprivation induced injury by inhibiting oxidative stress-mediated 5-lipoxygenase activation in PC12 cells. Acta Pharmacol. Sin. 2010, 31, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.P.; Zhu, L.L.; Zhao, T.; Zhao, H.Q.; Huang, X.; Ma, X.; Fan, M. Characteristics of Neural Stem Cells Expanded in Lowered Oxygen and the Potential Role of Hypoxia-Inducible Factor-1Alpha. NeuroSignals 2007, 15, 259–265. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| NO. | δH (J, Hz ) a | δc | NO. | δH (J, Hz ) a | δc |
|---|---|---|---|---|---|
| 2 | 164.0 | 2′′ | 5.51 (1H, dd, J = 13.1, 2.8 Hz) | 78.0 | |
| 3 | 6.76 (1H, s) | 102.7 | 3′′ | 3.31 (1H, dd, J = 17.1, 13.1 Hz) 2.60 (1H, dd, J = 17.1, 2.8 Hz) | 41.2 |
| 4 | 181.9 | 4′′ | 196.2 | ||
| 5 | 161.3 | 5′′ | 160.5 | ||
| 6 | 6.22(1H, d, J = 2.0 Hz) | 98.8 | 6′′ | 6.05 (1H, s) | 95.4 |
| 7 | 164.1 | 7′′ | 164.2 | ||
| 8 | 6.54 (1H, d, J = 2.0 Hz) | 94.0 | 8′′ | 104.6 | |
| 9 | 157.3 | 9′′ | 162.0 | ||
| 10 | 103.8 | 10′′ | 101.1 | ||
| 1′ | 122.0 | 1′′′ | 129.6 | ||
| 2′ | 7.90 (1H, d, J = 2.2 Hz) | 131.1 | 2′′′/6′′′ | 7.15 (2H, d, J = 8.7 Hz) | 127.5 |
| 3′ | 122.5 | 4′′′(-OH) | 9.51 (1H, br s) | 157.1 | |
| 4′ | 161.3 | 3′′′/5′′′ | 6.60 (2H, d, J = 8.7 Hz) | 114.6 | |
| 5′ | 7.15 (1H, d, J = 8.7 Hz) | 111.2 | |||
| 6′ | 7.96 (1H, dd, J = 8.7, 2.2 Hz) | 127.3 | |||
| OMe-4′ | 3.72 (3H, s) | 55.6 |
| Compounds | Promoted Survival Rate (%) | ||
|---|---|---|---|
| 45 μmol/L | 90 μmol/L | 180 μmol/L | |
| Blank control | 17.81 ± 1.34 | ||
| Negative control | 16.56 ±1.66 | 17.69 ± 1.68 | 15.12 ± 1.87 |
| Baicalin | 21.53 ±1.17 ** | 26.24 ±2.08 ** | 39.30 ± 2.31 ** |
| 1 | 20.88 ±1.41 * | 25.92 ±2.23 ** | 31.53 ± 2.39 ** |
| 2 | 18.07 ± 1.83 | 23.56 ±2.64 ** | 30.52 ± 2.49 ** |
| 3 | 17.27 ±1.61 | 24.15 ± 1.76 ** | 28.02 ± 2.45 ** |
| 4 | 18.24 ±1.09 | 20.25± 1.47 ** | 27.89 ±1.57 * |
| 5 | 16.16 ±0.21 | 20.88 ±1.41 * | 25.67 ± 1.83 ** |
| 6 | 19.35 ± 1.53 | 24.71 ± 2.75 ** | 38.27 ±3.24 ** |
| 7 | 18.82 ± 1.23 | 21.07 ± 2.35 * | 25.76 ± 1.19 ** |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zheng, J.-X.; Zheng, Y.; Zhi, H.; Dai, Y.; Wang, N.-L.; Fang, Y.-X.; Du, Z.-Y.; Zhang, K.; Li, M.-M.; Wu, L.-Y.; et al. New 3′,8′′-Linked Biflavonoids from Selaginella uncinata Displaying Protective Effect against Anoxia. Molecules 2011, 16, 6206-6214. https://doi.org/10.3390/molecules16086206
Zheng J-X, Zheng Y, Zhi H, Dai Y, Wang N-L, Fang Y-X, Du Z-Y, Zhang K, Li M-M, Wu L-Y, et al. New 3′,8′′-Linked Biflavonoids from Selaginella uncinata Displaying Protective Effect against Anoxia. Molecules. 2011; 16(8):6206-6214. https://doi.org/10.3390/molecules16086206
Chicago/Turabian StyleZheng, Jun-Xia, Yang Zheng, Hui Zhi, Yi Dai, Nai-Li Wang, Yan-Xiong Fang, Zhi-Yun Du, Kun Zhang, Ming-Ming Li, Li-Ying Wu, and et al. 2011. "New 3′,8′′-Linked Biflavonoids from Selaginella uncinata Displaying Protective Effect against Anoxia" Molecules 16, no. 8: 6206-6214. https://doi.org/10.3390/molecules16086206
APA StyleZheng, J.-X., Zheng, Y., Zhi, H., Dai, Y., Wang, N.-L., Fang, Y.-X., Du, Z.-Y., Zhang, K., Li, M.-M., Wu, L.-Y., & Fan, M. (2011). New 3′,8′′-Linked Biflavonoids from Selaginella uncinata Displaying Protective Effect against Anoxia. Molecules, 16(8), 6206-6214. https://doi.org/10.3390/molecules16086206