3.1. Chemistry
3.1.1. General
Melting points were determined on a Gallenkamp apparatus and are uncorrected. IR spectra were recorded in a Pye-Unicam SP300 instrument in potassium bromide discs.
1H-NMR spectra were recorded in a Varian Mercury VXR-300 spectrometer at 300 MHz in DMSO-
d6 and the chemical shifts were related to TMS as standard solvent. Mass spectra were recorded in a GCMS-QP 1000 EX Shimadzu spectrometer, the ionizing voltage was 70 eV. Elemental analyses were carried out at the Microanalytical Laboratory of Cairo University, Giza, Egypt. Antitumor activity was evaluated by the Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt. 3-Amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3
H-chromeno[2,3-d]pyrimidine (
1) [
10] and hydrazonoyl halides
2 [
26,
27] were prepared as reported in the literature.
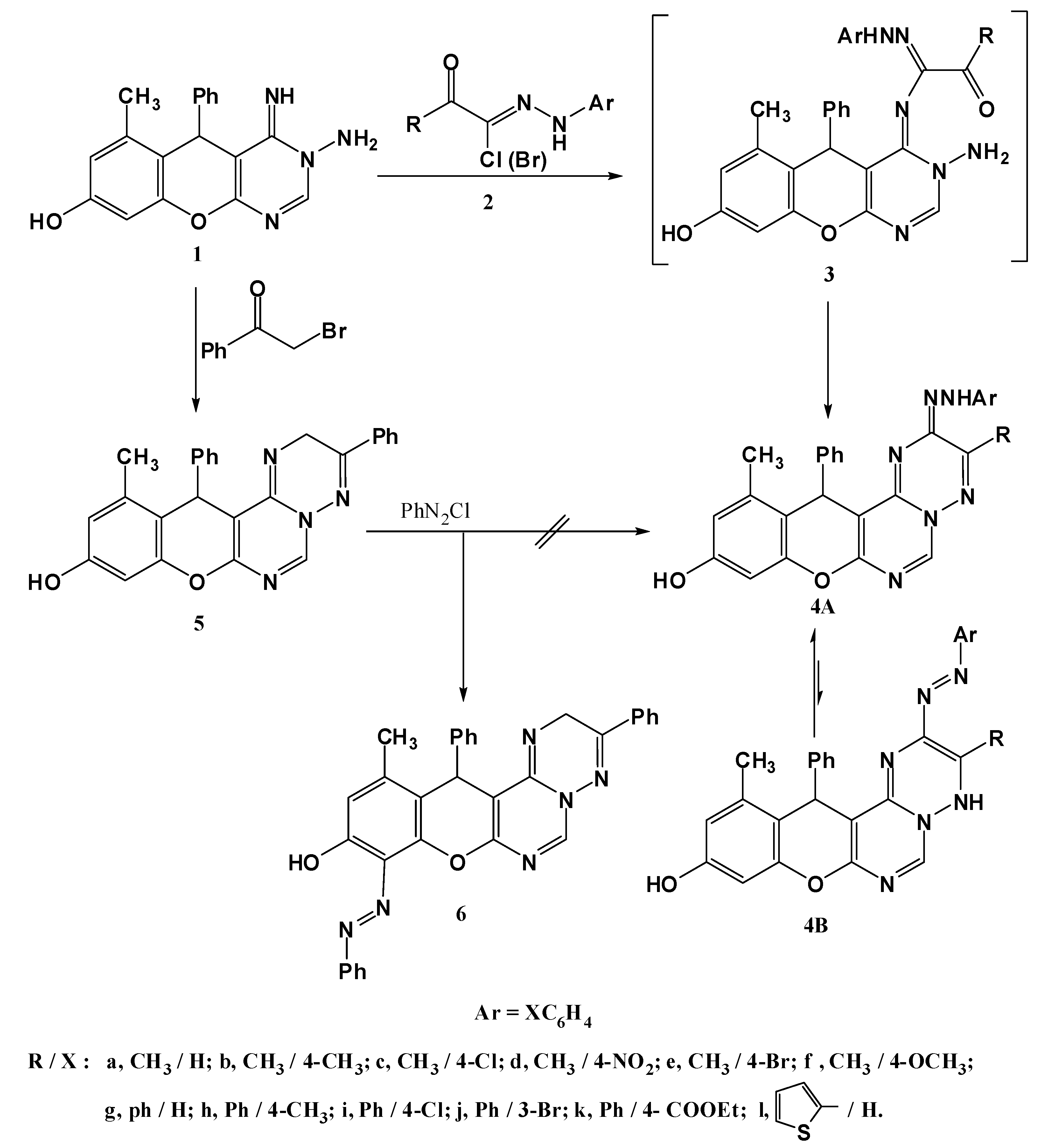
3.1.2. Synthesis of 10-Hydroxy-3-substituted-12-methyl-13-phenyl-2-(2-substituted phenyl-hydrazono)-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazines 4a–l
General procedure: A mixture of 3-amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno[2,3-d] pyrimidine (1, 0.32 g, 1 mmol) and the appropriate hydrazonoyl halide 2 (1 mmol) in ethanol (20 mL) was refluxed for 2 h (monitored by TLC), then allowed to cool and the solid formed was filtered off, washed with ethanol, dried and recrystallized from DMF to give 4a–l.
10-Hydroxy-3,12-dimethyl-13-phenyl-2-(2-phenylhydrazono)-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4a). Yield 71%; reddish-brown solid; mp. 334 °C; IR (KBr): v 1630 (C=N), 3427 (br, OH, NH) cm−1; 1H-NMR (DMSO-d6): δH 2.14 (3H, s, 12-CH3), 2.16 (3H, s, 3-CH3), 5.40 (1H, s, 13-H), 6.45 (1H, s, 9-H), 6.52 (1H, s, 11-H), 6.75–7.45 (10H, m, Ar-H), 8.39 (1H, s, 6-H), 9.30 (1H, br s, NH), 9.66 (1H, s, OH); MS m/z (%): 463 (M+ + 1, 38), 462 (M+, 100), 385 (23), 192 (24), 77 (33). Anal. Calcd for C27H22N6O2 (462.18): C, 70.12; H, 4.79; N, 18.17. Found C, 70.10; H, 4.65; N, 18.03%.
10-Hydroxy-3,12-dimethyl-13-phenyl-2-[2-(p-tolyl)hydrazono]-2H,13H-chromeno[2,3-d]-pyrimido[1,6-b][1,2,4] triazine (4b). Yield 74%; reddish-brown solid; mp. 345 °C; IR (KBr): v 1639 (C=N), 3421 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.18 (3H, s, 12-CH3), 2.19 (3H, s, p-toly-CH3), 2.20 (3H, s, 3-CH3), 5.44 (1H, s, 13-H), 6.48 (1H, s, 9-H), 6.56 (1H, s, 11-H), 6.82–7.55 (9H, m, Ar-H), 8.42 (1H, s, 6-H), 9.37 (1H, br s, NH), 9.72 (1H, s, OH); MS m/z (%): 477 (M+ + 1, 34), 476 (M+, 100), 399 (14), 253 (18), 200 (22), 77 (28). Anal. Calcd for C28H24N6O2 (476.20): C, 70.57; H, 5.08; N, 17.64. Found C, 70.51; H, 5.11; N, 17.34%.
2-[2-(4-Chlorophenyl)hydrazono]-10-Hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4c). Yield 76%; reddish-brown solid; mp. 352 °C; IR (KBr): v 1639 (C=N), 3448 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.19 (3H, s, 12-CH3), 2.21 (3H, s, 3-CH3), 5.42 (1H, s, 13-H), 6.49 (1H, s, 9-H), 6.58 (1H, s, 11-H), 6.88–7.63 (9H, m, Ar-H), 8.40 (1H, s, 6-H), 9.39 (1H, br s, NH), 9.75 (1H, s, OH); MS m/z (%): 498 (M+ + 2, 36), 497 (M+ + 1, 23), 496 (M+, 100), 428 (27), 253 (18), 209 (46), 77 (60), 55 (73). Anal. Calcd for C27H21ClN6O2 (496.14): C, 65.26; H, 4.26; N, 16.91. Found C, 65.10; H, 4.34; N, 16.69%.
10-Hydroxy-3,12-dimethyl-2-[2-(4-nitrophenyl)hydrazono]-13-phenyl--2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4d). Yield 76%; dark red solid; mp. 358 °C; IR (KBr): v 1639 (C=N), 3440 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.20 (3H, s, 12-CH3), 2.21 (3H, s, 3-CH3), 5.48 (1H, s, 13-H), 6.48 (1H, s, 9-H), 6.60 (1H, s, 11-H), 6.89–7.77 (9H, m, Ar-H), 8.43 (1H, s, 6-H), 9.42 (1H, br s, NH), 9.77 (1H, s, OH); MS m/z (%): 508 (M+ + 1, 17), 507 (M+, 46), 429 (20), 294 (37), 253 (50), 77 (44), 55 (100). Anal. Calcd for C27H21N7O4 (507.17): C, 63.90; H, 4.17; N, 19.32. Found C, 63.76; H, 4.02; N, 19.12%.
2-[2-(4-Bromophenyl)hydrazono]-10-Hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4e). Yield 71%; dark reddish-brown solid; mp. 358 °C; IR (KBr): v 1633 (C=N), 3452 (br, OH, NH) cm−1; 1H-NMR (DMSO-d6): δH 2.18 (3H, s, 12-CH3), 2.20 (3H, s, 3-CH3), 5.43 (1H, s, 13-H), 6.51 (1H, s, 9-H), 6.60 (1H, s, 11-H), 6.90–7.69 (9H, m, Ar-H), 8.41 (1H, s, 6-H), 9.38 (1H, br s, NH), 9.69 (1H, s, OH); MS m/z (%): 542 (M+ + 2, 93), 541 (M+ + 1, 71), 540 (M+, 100),463 (13), 253 (25), 90 (40), 77 (9). Anal. Calcd for C27H21BrN6O2 (540.09): C, 59.90; H, 3.91; N, 15.52. Found C, 59.70; H, 3.86; N, 15.37.%.
10-Hydroxy-2-[2-(4-methoxyphenyl)hydrazono]-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4f). Yield 74%; dark red solid; mp. 320 °C; IR (KBr): v 1638 (C=N), 3412 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.12 (3H, s, 12-CH3), 2.15 (3H, s, 3-CH3), 3.71 (3H, s, OCH3), 5.37 (1H, s, 13-H), 6.45 (1H, s, 9-H), 6.51 (1H, s, 11-H), 6.86–7.44 (9H, m, Ar-H), 8.35 (1H, s, 6-H), 9.14 (1H, br s, NH), 9.65 (1H, s, OH); MS m/z (%): 493 (M+ + 1, 33), 492 (M+, 100), 415 (10), 253 (15), 122 (31), 77 (22). Anal. Calcd for C28H24N6O3 (492.19): C, 68.28; H, 4.91; N, 17.06. Found C, 68.11; H, 4.78; N, 16.89%.
10-Hydroxy-12-methyl-3,13-diphenyl-2-(2-phenylhydrazono)-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4g). Yield 70%; orange solid; mp. 316 °C; IR (KBr): v 1637 (C=N), 3438 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.19 (3H, s, 12-CH3), 5.47 (1H, s, 13-H), 6.51 (1H, s, 9-H), 6.59 (1H, s, 11-H), 6.73–7.85 (15H, m, Ar-H), 8.47 (1H, s, 6-H), 9.48 (1H, br s, NH), 9.74 (1H, s, OH); MS m/z (%): 524 (M+, 16), 384 (12), 228 (100), 77 (20). Anal. Calcd for C32H24N6O2(524.20): C, 73.27; H, 4.61; N, 16.02. Found C, 73.12; H, 4.45; N, 15.22%.
10-Hydroxy-12-methyl-3,13-diphenyl-2-[2(p-tolyl)hydrazono]-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4h). Yield 74%; dark brown solid; mp. 312 °C; IR (KBr): v 1628 (C=N), 3427 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.18 (3H, s, 12-CH3), 2.50 (3H, s, p-tolyl-CH3), 5.44 (1H, s, 13-H), 6.47 (1H, s, 9-H), 6.54 (1H, s, 11-H), 6.76–7.87 (14H, m, Ar-H), 8.42 (1H, s, 6-H), 9.44 (1H, br s, NH), 9.64 (1H, s, OH); MS m/z (%): 540 (M+ + 2, 9), 539 (M+ + 1, 42), 538 (M+, 100), 316 (10), 238 (17), 77 (27). Anal. Calcd for C33H26N6O2 (538.21): C, 73.59; H, 4.87; N, 15.60. Found C, 73.59; H, 4.87; N, 15.60%.
2-[2-(4-Chlorophenyl)hydrazono]-10-Hydroxy-12-methyl-3,13-diphenyl2H,13H-chromeno-[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4i). Yield 72%; dark brown solid; mp. 330 °C; IR (KBr): v 1643 (C=N), 3444 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.15 (3H, s, 12-CH3), 5.43 (1H, s, 13-H), 6.40 (1H, s, 9-H), 6.51 (1H, s, 11-H), 6.72–7.98 (14H, m, Ar-H), 8.43 (1H, s, 6-H), 9.45 (1H, br s, NH), 9.70 (1H, s, OH); MS m/z (%): 559 (M+ + 1, 9), 558 (M+, 21), 391 (12), 201 (21), 105 (47), 77 (47), 55 (100). Anal. Calcd for C32H23ClN6O2 (558.16): C, 68.75; H, 4.15; N, 15.03. Found C, 68.73; H, 4.10; N, 15.00%.
2-[2-(3-Bromophenyl)hydrazono]-10-Hydroxy-12-methyl-3,13-diphenyl-2H,13H-chromeno-[2,3-d]pyrimido[1,6-b][1,2,4] triazine (4j). Yield 70%; dark brown solid; mp. 180 °C; IR (KBr): v 1643 (C=N), 3413 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.17 (3H, s, 12-CH3), 5.46 (1H, s, 13-H), 6.48 (1H, s, 9-H), 6.56 (1H, s, 11-H), 6.69–7.90 (14H, m, Ar-H), 8.47 (1H, s, 6-H), 9.50 (1H, br s, NH), 9.74 (1H, s, OH); MS m/z (%): 604 (M+ + 2, 3), 603 (M+ + 1, 4), 602 (M+, 3), 503 (4), 471 (5), 305 (15), 228 (88), 105 (100), 77 (79). Anal. Calcd for C32H23BrN6O2 (602.11): C, 63.69; H, 3.84; N, 13.93. Found C, 63.57; H, 3.75; N, 13.68%.
Ethyl4-[10-hydroxy-12-methyl-3,13-diphenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazin-2-ylidene)hydrazinyl]benzoate (4k). Yield 72%; dark brown solid; mp. 352 °C; IR (KBr): v 1639 (C=N), 1715 (C=O), 3420 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 1.34 (3H, t, CH3), 2.21 (3H, s, 12-CH3), 4.51 (2H, q, CH2), 5.51 (1H, s, 13-H), 6.52 (1H, s, 9-H), 6.58 (1H, s, 11-H), 6.70–7.92 (14H, m, Ar-H), 8.40 (1H, s, 6-H), 9.51 (1H, br s, NH), 9.70 (1H, s, OH); MS m/z (%): 597 (M+ + 1, 13), 596 (M+, 24), 567 (100), 524 (30), 432 (18), 228 (49), 103 (78), 77 (66). Anal. Calcd for C35H28N6O4 (596.22): C, 70.46; H, 4.73; N, 14.09. Found C, 70.34; H, 4.45; N, 13.97%.
10-Hydroxy-12-methyl-13-phenyl-3-2-(2-phenylhydrazono)-(thiophen-2-yl)-2H,13H-chromeno2,3-d]pyrimido[1,6-b][1,2,4]triazine (4l). Yield 76%; dark red solid; mp. 228 °C; IR (KBr): v 1647 (C=N), 3387 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.19 (3H, s, 12-CH3), 5.46 (1H, s, 13-H), 6.48 (1H, s, 9-H), 6.56 (1H, s, 11-H), 6.68–7.96 (13H, m, Ar-H), 8.49 (1H, s, 6-H), 9.54 (1H, br s, NH), 9.71 (1H, s, OH); MS m/z (%): 531 (M+ + 1, 2), 530 (M+ , 7), 385 (9), 306 (39), 228 (100), 77 (29). Anal. Calcd for C30H22N6O2S (530.15): C, 67.91; H, 4.18; N, 15.84. Found C, 67.91; H, 4.18; N, 15.84%.
3.1.3. Synthesis of 10-hydroxy-12-methyl-9-phenylazo-2,13-diphenyl-13H-chromeno[2,3-d]pyrimido [1,6-b][1,2,4] triazine 6
Synthesis of 10-hydroxy-12-methyl-2,13-diphenyl-13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (5). A mixture of 1 (0.320 g, 1 mmol) and phenacyl bromide (0.198 g, 1 mmol) in absolute ethanol (30 mL) was refluxed for 2 h (monitored by TLC). The product started to separate out during the course of reaction. The crystalline solid was filtered, washed with water, dried and recrystallized from dioxane to give compound 5 in 76% yield as yellow solid; mp. 230 °C; IR (KBr): v 1632 (C=N), 3425 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.14 (3H, s, 12-CH3), 4.23 (2H, s, CH2), 5.55 (1H, s, 13-H), 6.52 (1H, s, 9-H), 6.54 (1H, s, 11-H), 7.18–7.38 (10H, m, Ar-H),), 8.63 (1H, s, 6-H), 9.82 (1H, s, OH); MS m/z (%): 421 (M+ +1, 1), 420 (M+, 2), 329 (14), 305 (16), 253 (21), 228 (100), 201 (23), 105 (15), 77 (26); Anal. Calcd for C26H20N4O2 (420.47): C, 74.27; H, 4.79; N, 13.32. Found C, 74.14; H, 4.87; N, 13.35%.
Coupling of 5 with benzenediazonium chloride. To a solution of 5 (0.421g, 1 mmol) in ethanol (20 mL) was added sodium acetate trihydrate (0.138 g, 1 mmol), and the mixture was cooled to 0–5 °Cin an ice bath. To the resulting cold solution was added portionwise a cold solution of benzenediazonium chloride (1 mmol) [prepared by diazotizing aniline] dissolved in hydrochloric acid (6 M, 1 mL) with a solution of sodium nitrite (0.07 g, 1 mmol) in water (2 mL). After complete addition of the diazonium salt, the reaction mixture was stirred for a further 30 min in an ice bath. The solid that separated was filtered off, washed with water and finally recrystallized from ethanol to give product 6. Yield 78%; orange solid; mp. 298 °C; IR (KBr): v 1634 (C=N), 3466 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.16 (3H, s, 12-CH3), 4.24 (2H, s, CH2), 5.56 (1H, s, 13-H), 6.54 (1H, s, 11-H), 7.03–7.41 (15H, m, Ar-H), 8.63 (1H, s, 6-H), 9.88 (1H, s, OH); MS m/z (%): 524 (M+, 23), 305 (100), 228 (87), 77 (64). Anal. Calcd for C32H24N6O2(524.20): C, 73.27; H, 4.61; N, 16.02. Found C, 73.10; H, 4.65; N, 15.12%.
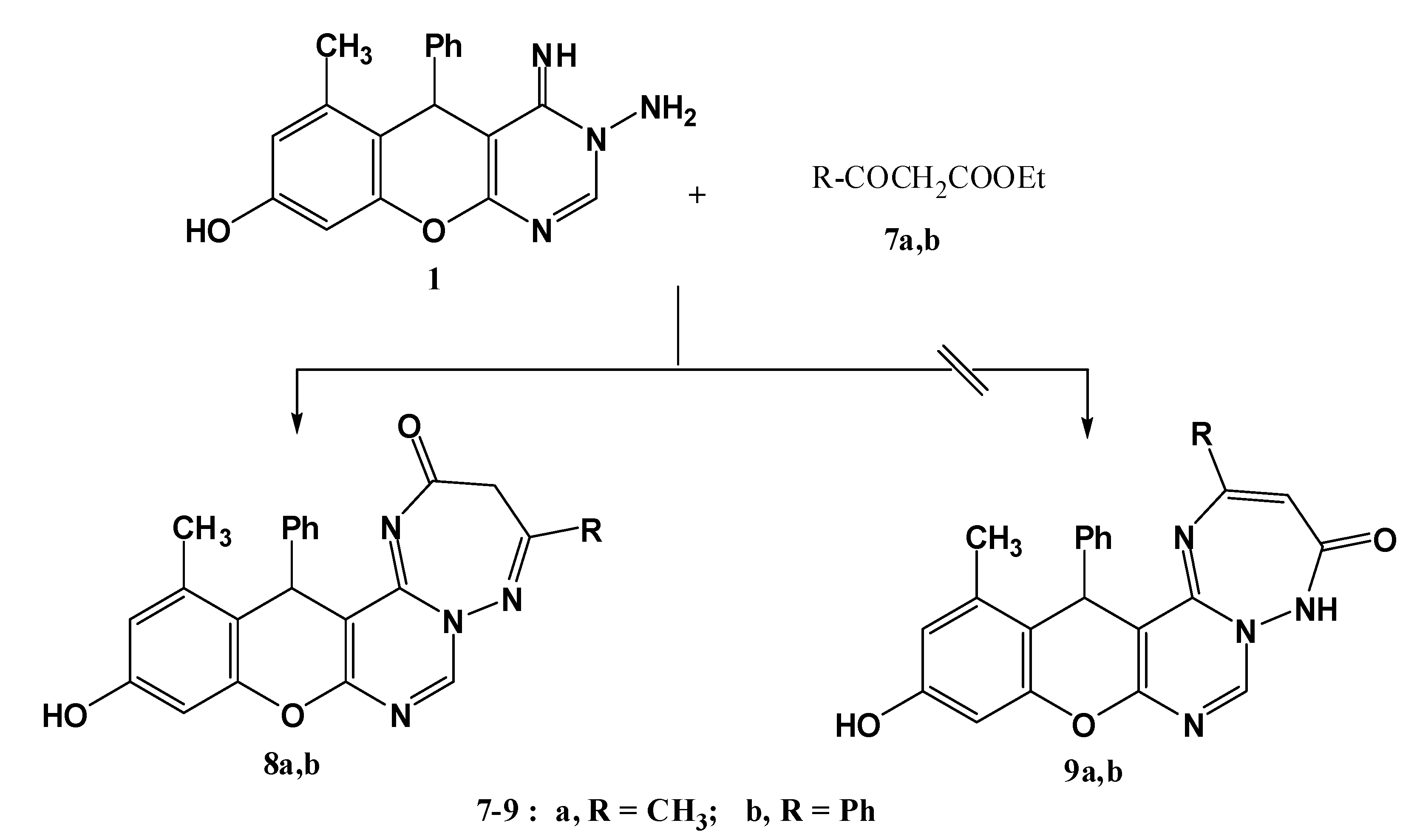
3.1.4. Synthesis of 4-substituted-11-hydroxy-13-methyl-14-phenyl-3H,14H-chromeno[2,3-d]pyrimido [1,6-b][1,2,4] triazepin-2(3H)-ones 8a,b.
A mixture of compound 1 (0.32 g, 1 mmol) and ethyl acetoacetate or ethyl benzoylacetate (1.5 mmol) was heated under reflux for 2 h. After cooling, the solid precipitated was collected and crystallized from dioxane to give 8a,b, respectively.
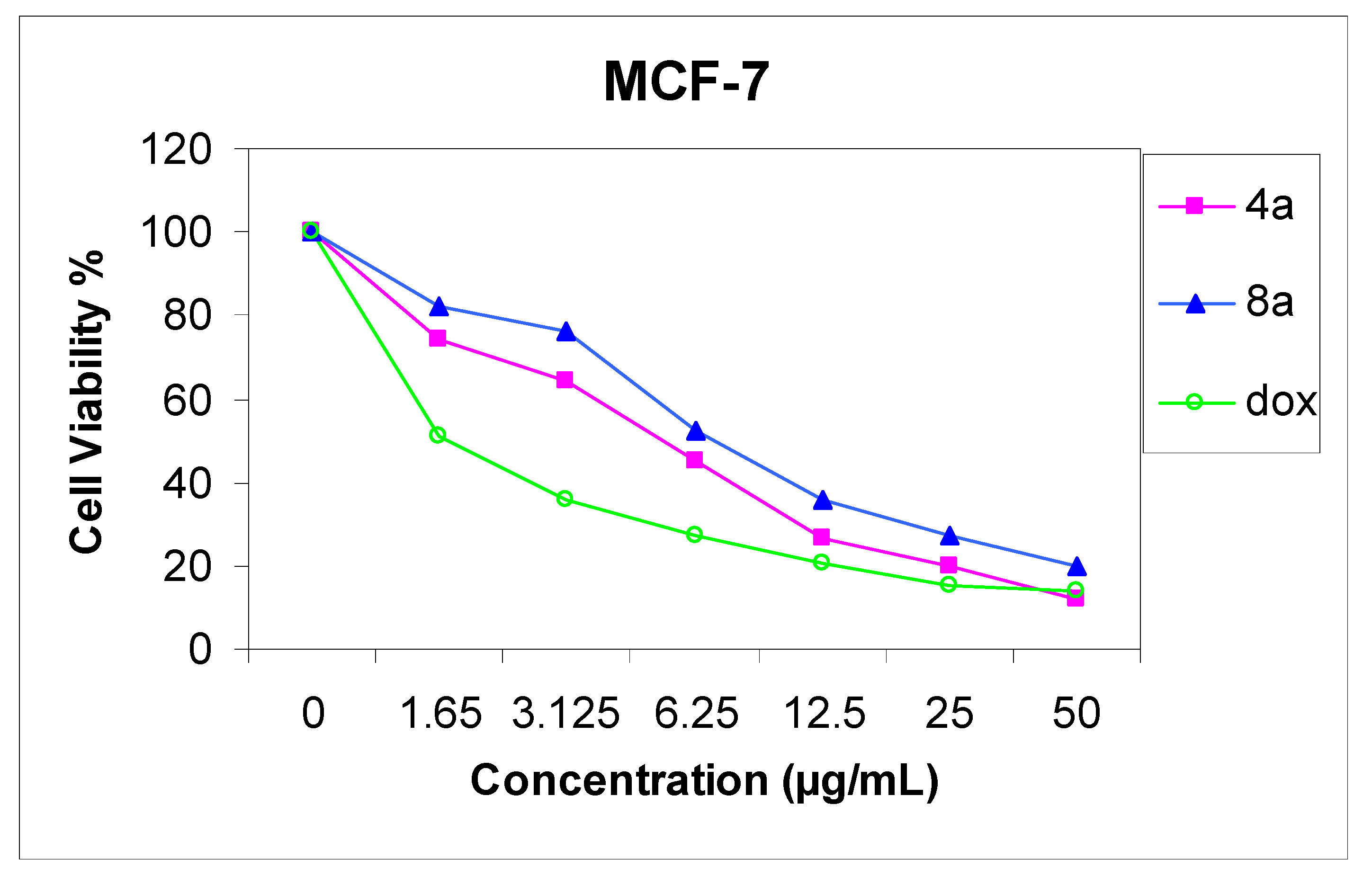
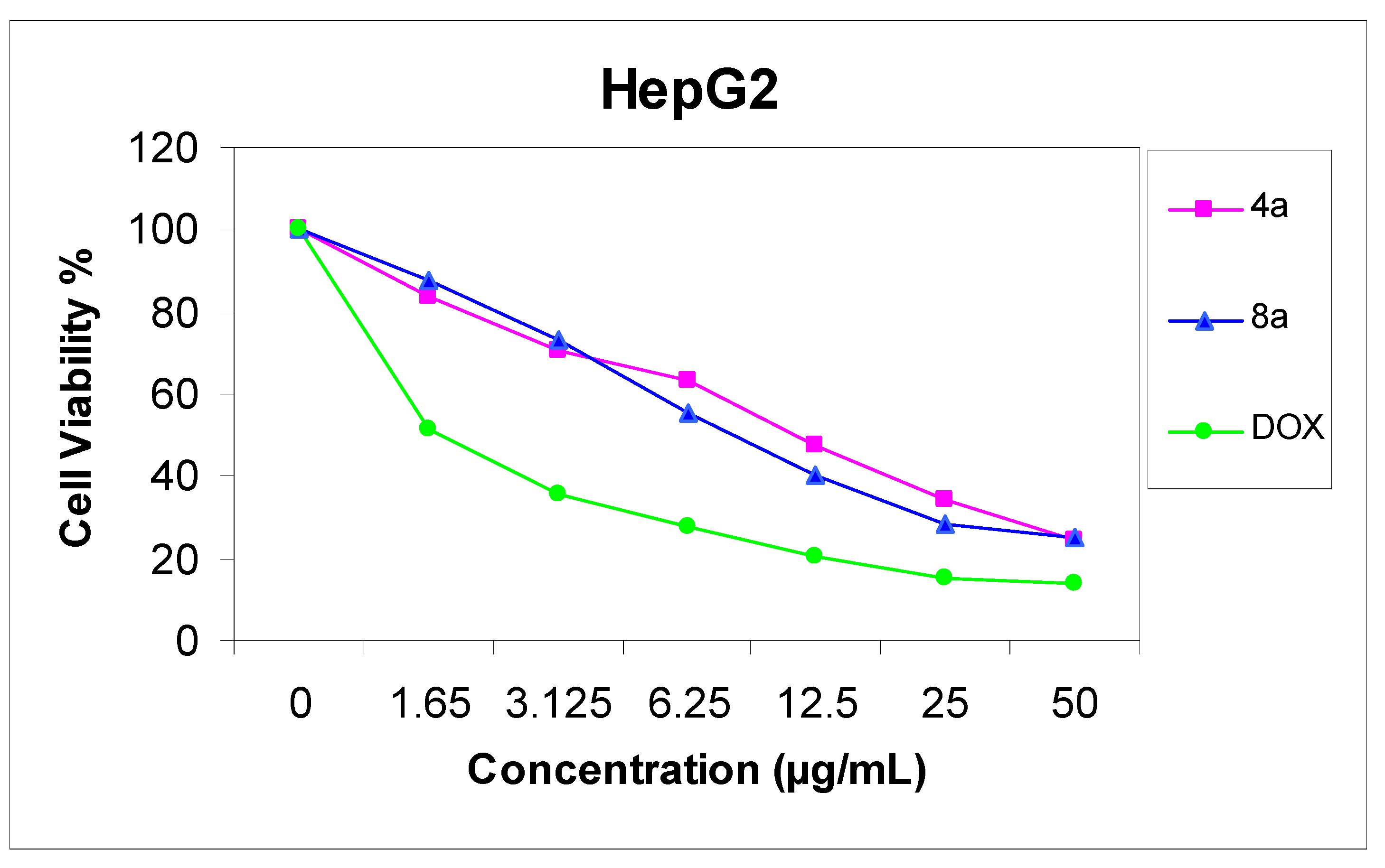
11-Hydroxy-4,13-dimethyl-14-phenyl-3H,14H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazepin-2(3H)-one (8a). Yield 74%; yellow solid; mp. 189 °C; IR (KBr): v 1635 (C=N), 1720 (CO), 3251 (NH), 3406 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.07 (3H, s, 13-CH3), 2.17 (3H, s, 4-CH3), 4.06 (2H, s, CH2), 5.57 (1H, s, 14-H), 6.50 (1H, s, 10-H), 6.60 (1H, s, 12-H), 7.14–7.29 (5H, m, Ar-H), 9.50 (1H, s, 7-H), 9.72 (1H, s, OH); MS m/z (%): 388 (M+ + 2, 1), 387 (M+ + 1, 5), 386 (M+, 16), 309 (100), 266 (19) 77 (11), 55. Anal. Calcd for C22H18N4O3 (386.14): C, 68.38; H, 4.70; N, 14.50. Found C, 68.32; H, 4.65; N, 14.36%.
11-Hydroxy-13-methyl-4,14-diphenyl-3H,14H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazepin-2(3H)-one (8b). Yield 74%; yellow solid; mp. 214 °C; IR (KBr): v 1632 (C=N), 1702 (CO), 3416 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.10 (3H, s, 13-CH3), 4.15 (2H, s, CH2), 5.62 (1H, s, 14-H), 6.55 (1H, s, 10-H), 6.68 (1H, s, 12-H), 7.06–7.67 (10H, m, Ar-H), 9.52 (1H, s, 7-H), 9.82 (1H, s, OH); MS m/z (%): 449 (M++1, 8), 448 (M+, 20), 371 (100), 266 (24), 105 (76), 77 (75). Anal. Calcd for C27H20N4O3 (448.15): C, 72.31; H, 4.49; N, 12.49. Found C, 72.18; H, 4.34; N, 12.29%.
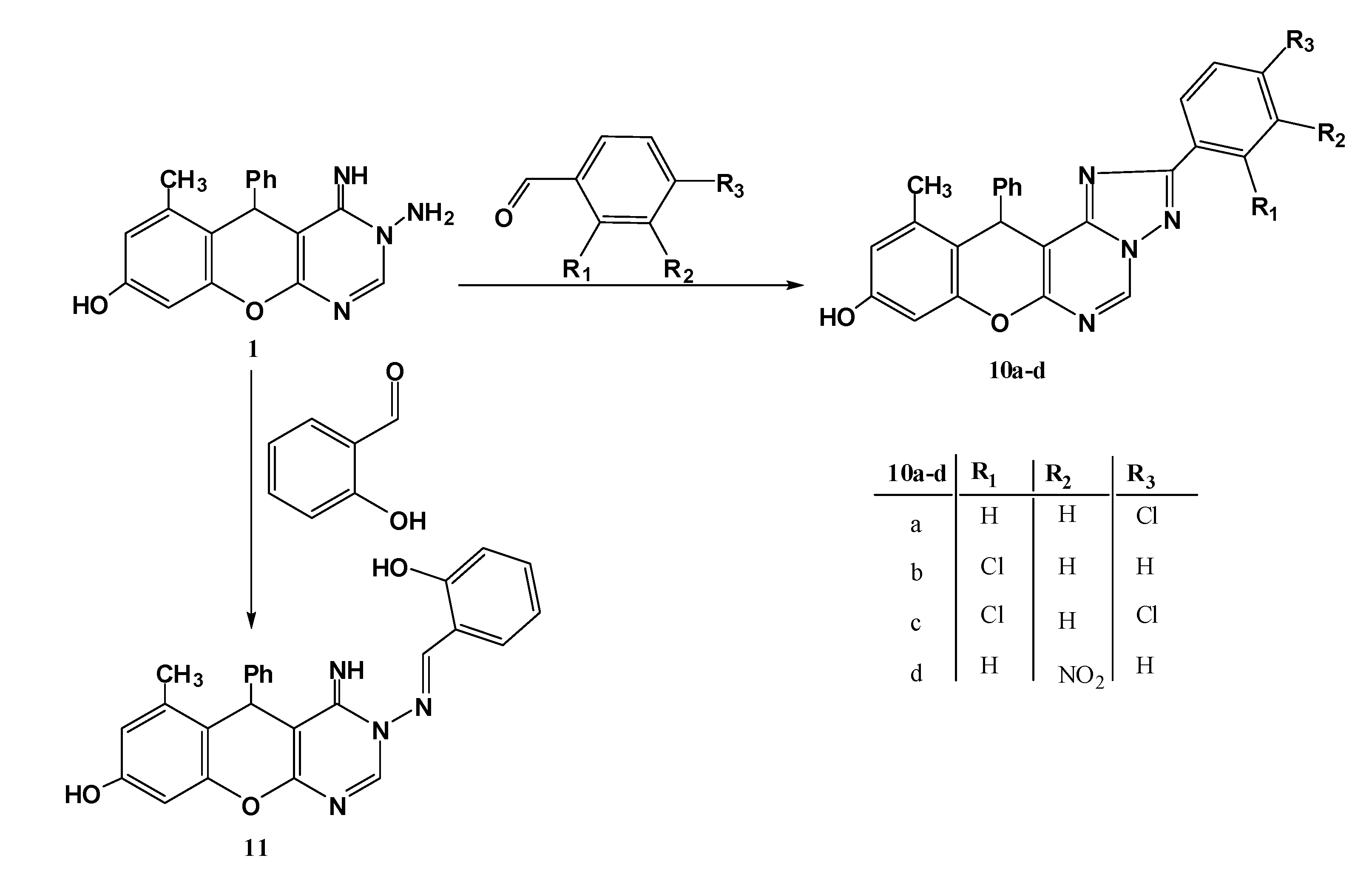
3.1.5. Reaction of N-aminopyrimidine 1 with Aromatic Aldehydes
General procedure: An appropriate aromatic aldehyde (1 mmol.) was added to a solution of the N-aminopyrimidine 1 (0.32 g, 1 mmol) in absolute ethanol (15 mL) containing a few drops of piperidine, the resulting mixture refluxed for 3 h. The solids formed after cooling were collected by filtration, washed with ether and crystallized from DMF.
9-Hydroxy-11-methyl-12-phenyl-2-(4-chlorophenyl)-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (10a). Yield 76%; yellow solid; mp. 270 °C; IR (KBr): v 1639 (C=N), 3441 (br, OH) cm−1; 1H-NMR (DMSO-d6): δH 2.20 (3H, s, 11-CH3), 6.01 (1H, s, 12-CH), 5.86 (1H, s, NH), 6.47 (1H, s, 8-H), 6.55 (1H, s, 10-H), 7.08–8.40 (9H, m, Ar-H), 9.67 (1H, s, 5-H), 11.36 (1H, s, OH); MS m/z (%): 440 (M+, 2), 366 (12), 305 (100), 228 (69), 201 (33), 138(73), 77(52). Anal.Calcd for C25H17ClN4O2S (440.10): C, 68.11; H, 3.89; N, 12.71. Found C, 68.01; H, 3.67; N, 12.56%.
2-(2-Chlorophenyl)-9-hydroxy-11-methyl-12-phenyl-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (10b). Yield 74%; yellow solid; mp. 252 °C; IR (KBr): v 1636 (C=N), 3438 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.21 (3H, s, 11-CH3), 6.02 (1H, s, 12-H), 6.48 (1H, s, 8-H), 6.52 (1H, s, 10-H), 7.05–8.33 (9H, m, Ar-H), 9.60 (1H, s, 5-H), 11.03 (1H, s, OH); MS m/z (%): 440 (M+, 12), 305 (66), 228 (100), 138(58), 77(47). Anal.Calcd for C25H17ClN4O2S (440.10): C, 68.11; H, 3.89; N, 12.71. Found C, 68.21; H, 3.71; N, 12.49%.
2-(2,4-Dichlorophenyl)-9-Hydroxy-11-methyl-12-phenyl-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (10c). Yield 74%; yellow solid; mp. 298 °C; IR (KBr): v 1639 (C=N), 3444 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.25 (3H, s, 11-CH3), 6.12 (1H, s, 12-H), 5.88 (1H, s, NH), 6.49 (1H, s, 8-H), 6.61 (1H, s, 10-H), 7.08–8.46 (8H, m, Ar-H), 9.74 (1H, s, 5-H), 11.36 (1H, s, OH); MS m/z (%): 476 (M+ + 2, 7), 474 (M+, 6), 397 (26), 305 (24), 228 (75), 145 (100), 77 (38). Anal. Calcd for C25H16Cl2N4O2 (474.07): C, 63.17; H, 3.39; N, 11.79. Found C, 63.10; H, 3.21; N, 11.53%.
9-Hydroxy-11-methyl-2-(3-nitrophenyl)-12-phenyl-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (10d). Yield 72%; yellow solid; mp. 240 °C; IR (KBr): v 1632 (C=N), 3412 (OH) cm−1; 1H-NMR (DMSO-d6): 2.09 (3H, s, CH3), 5.58 (1H, s, 11-H), 6.42 (1H, s, 8- H), 6.57 (1H, s, 10-H), 7.10–8.46 (9H, m, Ar-H), 9.50 (1H, s, 5-H), 9.72 (1H, br s, OH); MS m/z (%): 452 (M+ + 1, 6), 451 (M+, 17), 374 (100), 172 (13),77 (18). Anal.Calcd for C25H17N5O4 (451.13): C, 66.51; H, 3.80; N, 15.51. Found C, 66.34; H, 3.65; N, 15.41%.
3-(2-Hydroxybenzylideneamino)-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno[2,3-d]pyrimidin-8-ol (11). Yield 78%; cannary yellow solid; mp. 192 °C; IR (KBr): v 1616 (C=N), 3433 (very br, 2OH,NH) cm−1; 1H-NMR (DMSO-d6): 1H-NMR δH 2.19 (3H, s, 6-CH3), 5.68 (1H, s, 5-H), 6.45 (1H, s, 9-H), 6.55 (1H, s, 7-H), 6.89–7.56 (9H, m, Ar-H), 8.27 (1H, s, CH=N-N), 8.37 (1H, s, 2-H), 8.53 (1H, s, NH), 8.57 (1H, s, OH); MS m/z (%):424 (M+, 29), 304 (100), 228 (94), 173 (35), 105 (29), 77 (75); Anal.Calcd for C25H20N4O3 (424.15): C, 70.74; H, 4.75; N, 13.20. Found C, 70.31; H, 4.56; N, 13.02%.
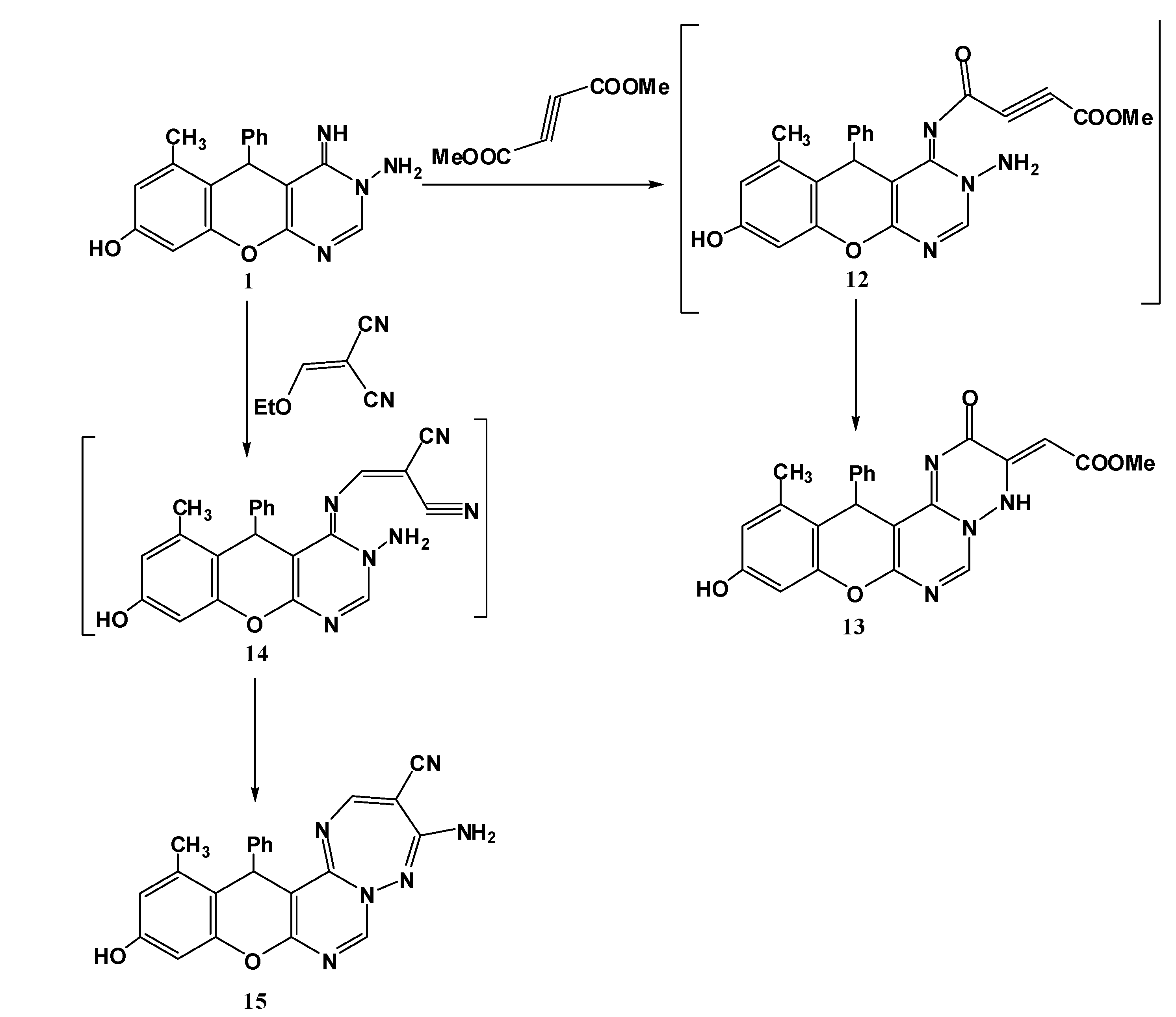
3.1.6. Reaction of 1 with Activated Unsaturated Compounds
Synthesis of 10-hydroxy-12-methyl-2-oxo-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazin-3(4H)-ylidene)acetate (13). An equimolar mixture of 1 (0.32 g, 1 mmol) and dimethylacetylene dicarboxylate (0.142 g, 1 mmol) in methanol (20 mL) was refluxed for 2 h (monitored by TLC). The formed solid was collected by filtration and recrystallized from DMF to give compound 13. Yield 78%; canary yellow solid; mp. 184 °C; IR (KBr): v = 1633 (C=N), 1665, 1712 (2C=O), 3466 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.17 (3H, s, 12-CH3), 3.48 (3H, s, OCH3), 5.42 (1H, s, 13-H), 6.60 (1H, s, CH=CO2Me), 6.52 (1H, s, 9-H), 6.62 (1H, s, 11-H), 7.11–7.34 (5H, m, Ar-H), 9.12 (1H, s, 6-H), 9.95 (1H, s, NH), 10.15 (1H, s, OH); MS m/z (%): 432 (M+ + 2, 2), 431 (M+ + 1, 2), 430 (M+, 5), 305 (22), 253 (25), 228 (100), 105 (69), 77 (62). Anal. Calcd for C23H18N4O5 (430.13): C, 64.18; H, 4.22; N, 13.02. Found C, 64.12; H, 4.13; N, 12.82%.
Synthesis of 4-Amino-11-hydroxy-13-methyl-14-phenyl-3H,14H-chromeno[2,3-d]pyrimido[1,6-b]1,2,4]triazepine-3-carbonitrile (15). A mixture of 1 (0.32 g, 1 mmol) and ethoxymethylene malononitrile (0.122 g, 1 mmol) in methanol (20 mL) was refluxed for 1 h (monitored by TLC). The reaction mixture was cooled and the resulting precipitate was filtered off and recrystallized from DMF/EtOH to give 15. Yield 76%; yellow solid; mp. 330 °C; IR (KBr): v 1628 (C=N), 2197 (CN), 3214, 3180 (NH2), 3466 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.07 (3H, s, 13-CH3), 5.61 (1H, s, 14-H), 6.50 (1H, s, 10-H), 6.60 (1H, s, 12-H), 7.12–7.33 (7H, m, Ar-H + NH2), 8.57 (1H, s, 2-H), 9.57 (1H, s, 7-H), 9.72 (1H, s, OH); MS m/z (%): 396 (M+, 3), 330 (13), 253 (100), 77 (11). Anal. Calcd for C22H16N6O2 (396.13): C, 66.66; H, 4.07; N, 21.20. Found C, 66.38; H, 4.01; N, 21.03%.
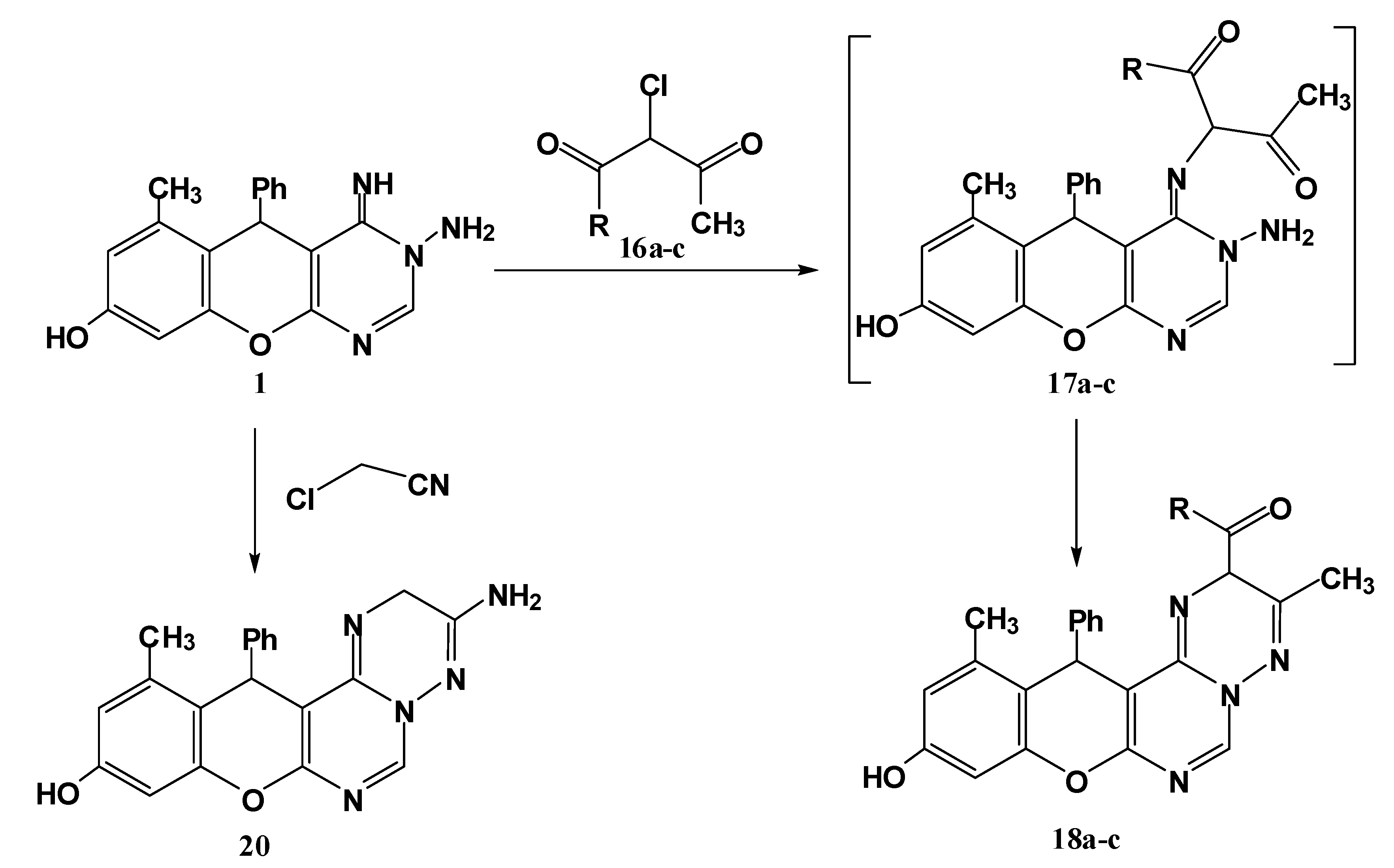
3.1.7. Reaction of 1 with Active Chloromethylene Compounds 16a–c and Chloroacetonitrile
General procedure: To a solution of 1 (0.32 g, 1 mmol) in ethanol was added triethylamine (0.7 mL) and the mixture was stirred for 10 min at room temperature. To the resulting clear solution was added active chloromethylene compounds 16a–c and chloroacetonitrile (1 mmol) dropwise while stirring the reaction mixture. After complete addition, the reaction mixture was refluxed for 2 h (monitored by TLC). The solid that precipitated was filtered off, washed with H2O, dried and finally crystallized from ethanol to give the respective 18a–c and 20.
2-Acetyl-10-hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (18a). Yield 74%; yellow solid; mp. 212 °C; IR (KBr): v 1639 (C=N), 1698 (C=O), 3410 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.17 (3H, s, 12-CH3), 2.19 (3H, s, 3-CH3), 2.25 (3H, s, CH3CO), 5.48 (1H, s, 2-H), 5.6 (1H, s, 13-H), 6.46 (1H, s, 9-H), 6.58 (1H, s, 11-H), 7.14–7.54 (5H, m, Ar-H), 8.20 (1H, s, 6-H), 9.69 (1H, br s, OH); MS m/z (%): 400 (M+, 8), 305 (12), 276 (100), 253 (11), 228 (92), 105 (18), 77 (15). Anal. Calcd for C23H20N4O3 (400.15): C, 68.99; H, 5.03; N, 13.99. Found C, 68.78; H, 4.83; N, 13.86%.
Ethyl-10-hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine-2-carboxylate (18b). Yield 74%; yellow solid; mp. 160 °C; IR (KBr): v 1634 (C=N), 1712 (C=O), 3422 (OH) cm−1; 1H-NMR (DMSO-d6): δH 1.30 (3H, t, CH3), 2.21 (3H, s, 12-CH3), 2.23 (3H, s, 3-CH3), 4.22 (2H, q, CH2), 5.50 (1H, s, 2-H), 5.58 (1H, s, 13-H), 6.40 (1H, s, 9-H), 6.49 (1H, s, 11-H), 7.11–7.36 (5H, m, Ar-H), 8.17 (1H, s, 6-H), 9.61 (1H, br s, OH); MS m/z (%): 430 (M+, 12), 354 (16), 305 (59), 268 (43), 228 (100), 105 (18), 76 (52). Anal. Calcd for C24H22N4O4 (430.16): C, 66.97; H, 5.15; N, 13.02. Found C, 66.76; H, 5.01; N, 12.92%.
10-Hydroxy-3,12-dimethyl-N,13-diphenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4] triazine-2-carboxamide (18c). Yield 76%; yellow solid; mp. 198 °C; IR (KBr): v 1636 (C=N), 1660 (C=O), 3433 (br, OH and NH) cm−1. 1H-NMR (DMSO-d6): δH 2.14 (3H, s, 12-CH3), 2.17 (3H, s, 3-CH3), 5.37 (1H, s, 2-H), 5.59 (1H, s, 13-H), 6.40 (1H, s, 9-H), 6.52 (1H, s, 11-H), 7.19–7.84 (10H, m, Ar-H), 8.18 (1H, s, 6-H), 9.45 (1H, s, NH), 9.79 (1H, br s, OH); MS m/z (%): 477 (M+, 16), 305 (100), 268 (43), 228 (94), 105 (46), 77 (68). Anal. Calcd for C28H23N5O3 (477.18): C, 70.43; H, 4.85; N, 14.67. Found C, 70.33; H, 4.74; N, 14.62%.
3-Amino-10-hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (20). Yield 73%; yellow solid; mp. 165 °C; IR (KBr): v 1639 (C=N), 3356, 3198 (NH2), 3406 (OH) cm−1. 1H-NMR (DMSO-d6): δH 2.11 (3H, s, 12-CH3), 3.88 (2H, s, CH2), 5.57 (1H, s, 13-H), 6.35 (2H, br s, NH2), 6.52 (1H, s, 9-H), 6.50 (1H, s, 11-H), 7.11–7.42 (5H, m, Ar-H), 8.67 (1H, s, 6-H), 9.84 (1H, s, OH); MS m/z (%): 359 (M+, 3), 253 (25), 228 (100), 77 (23). Anal. Calcd for C20H17N5O2 (359.14): C, 66.84; H, 4.77; N, 19.49. Found C, 66.67; H, 4.54; N, 19.36%.