Identification of Three New N-Demethylated and O-Demethyled Bisbenzylisoquinoline Alkaloid Metabolites of Isoliensinine from Dog Hepatic Microsomes
Abstract
:1. Introduction
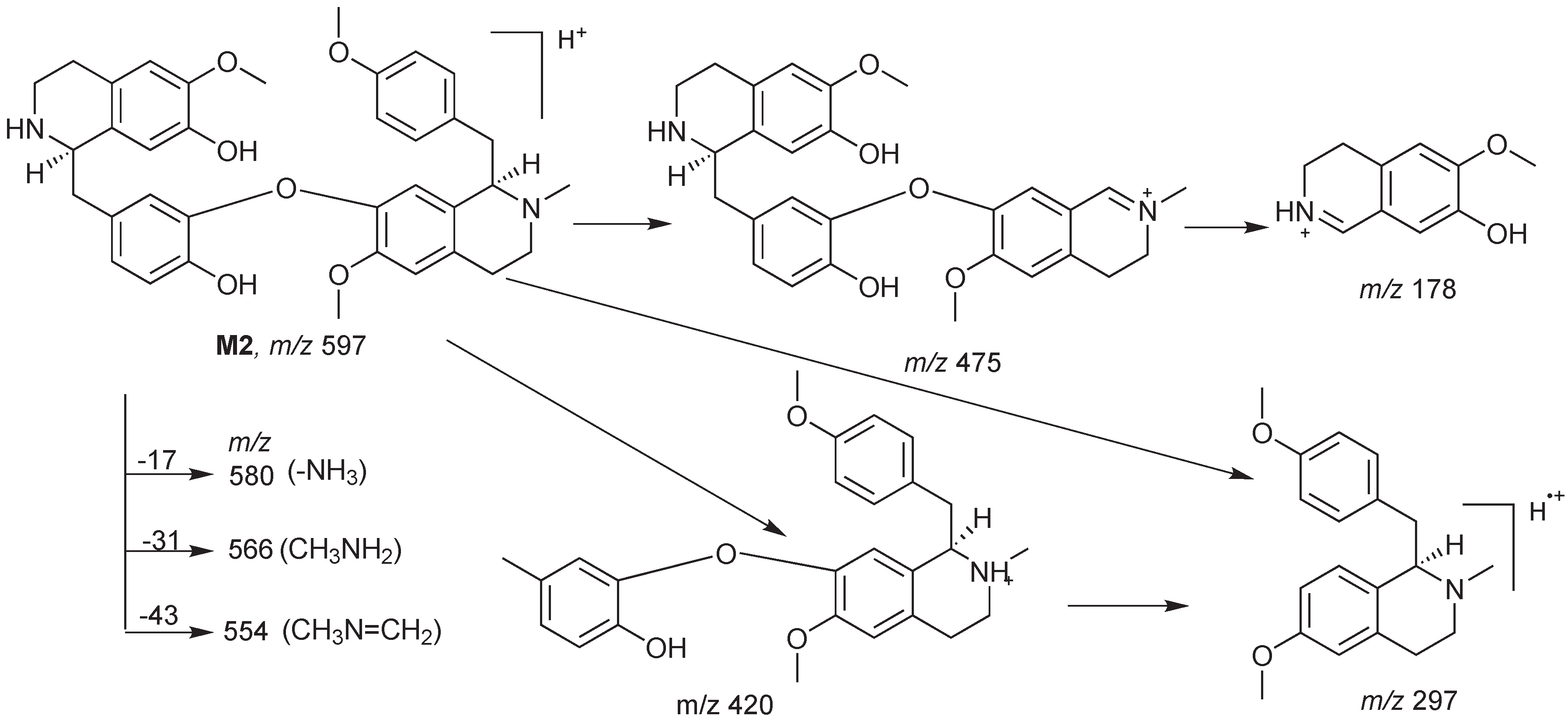
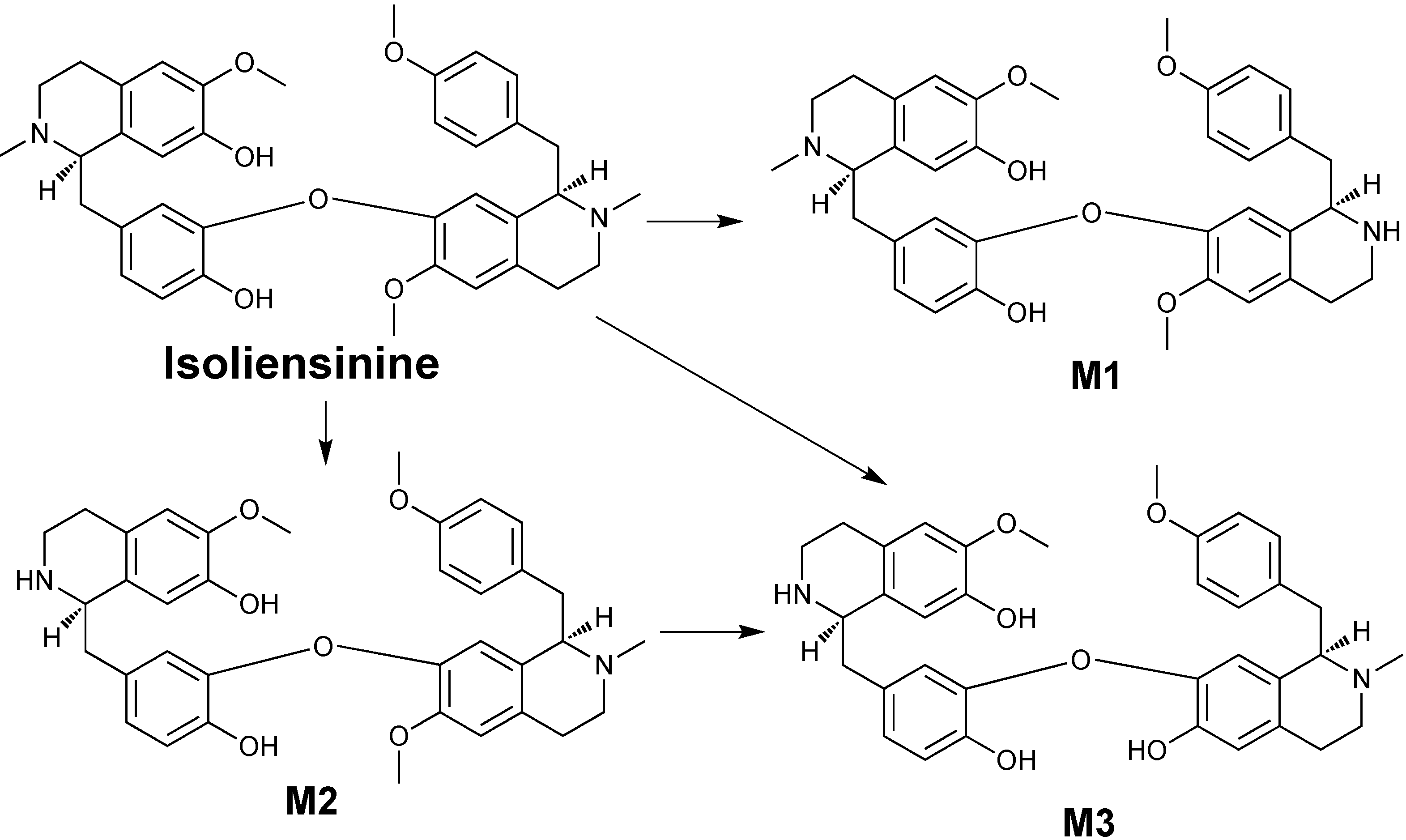
2. Results and Discussion
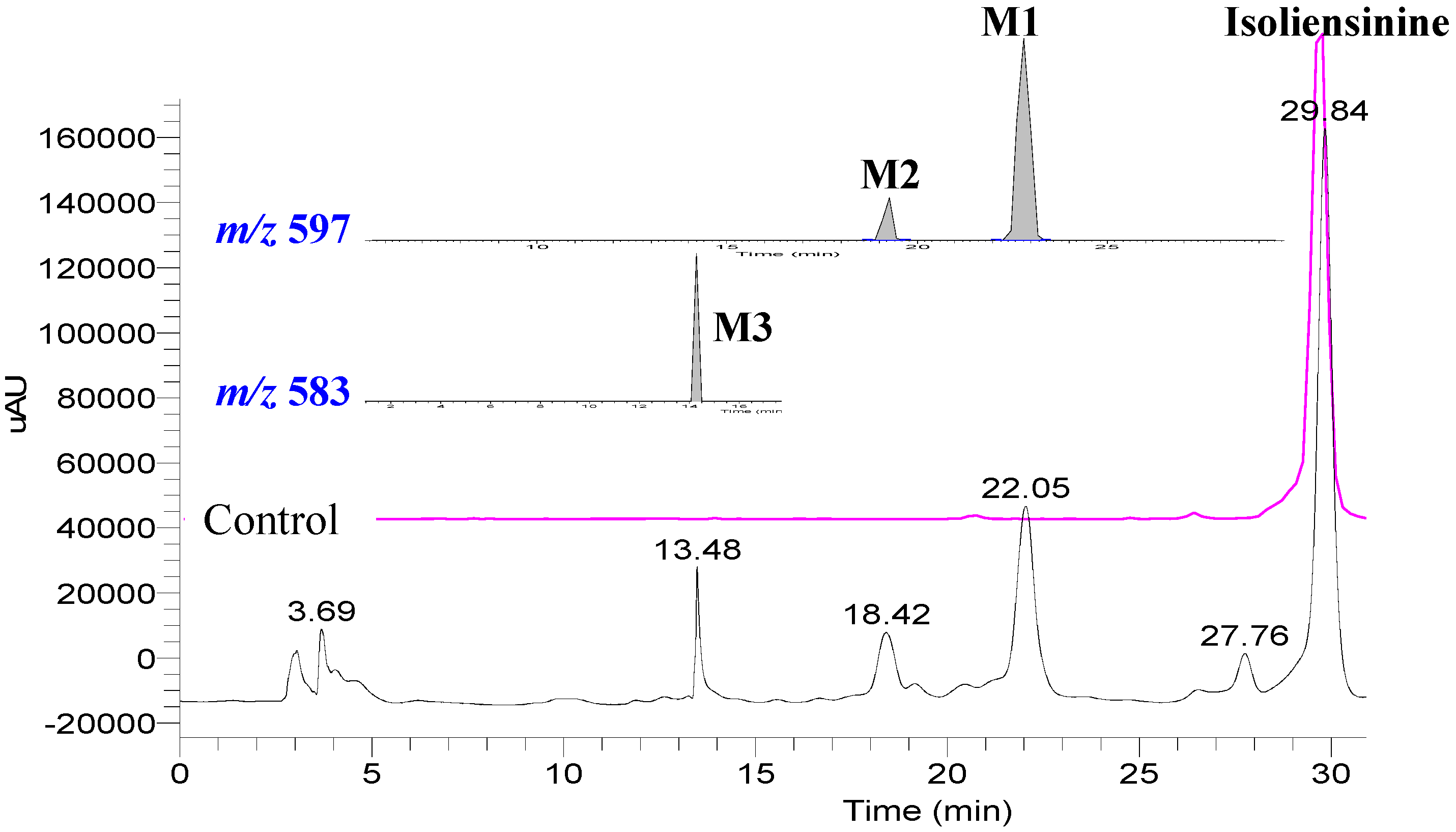
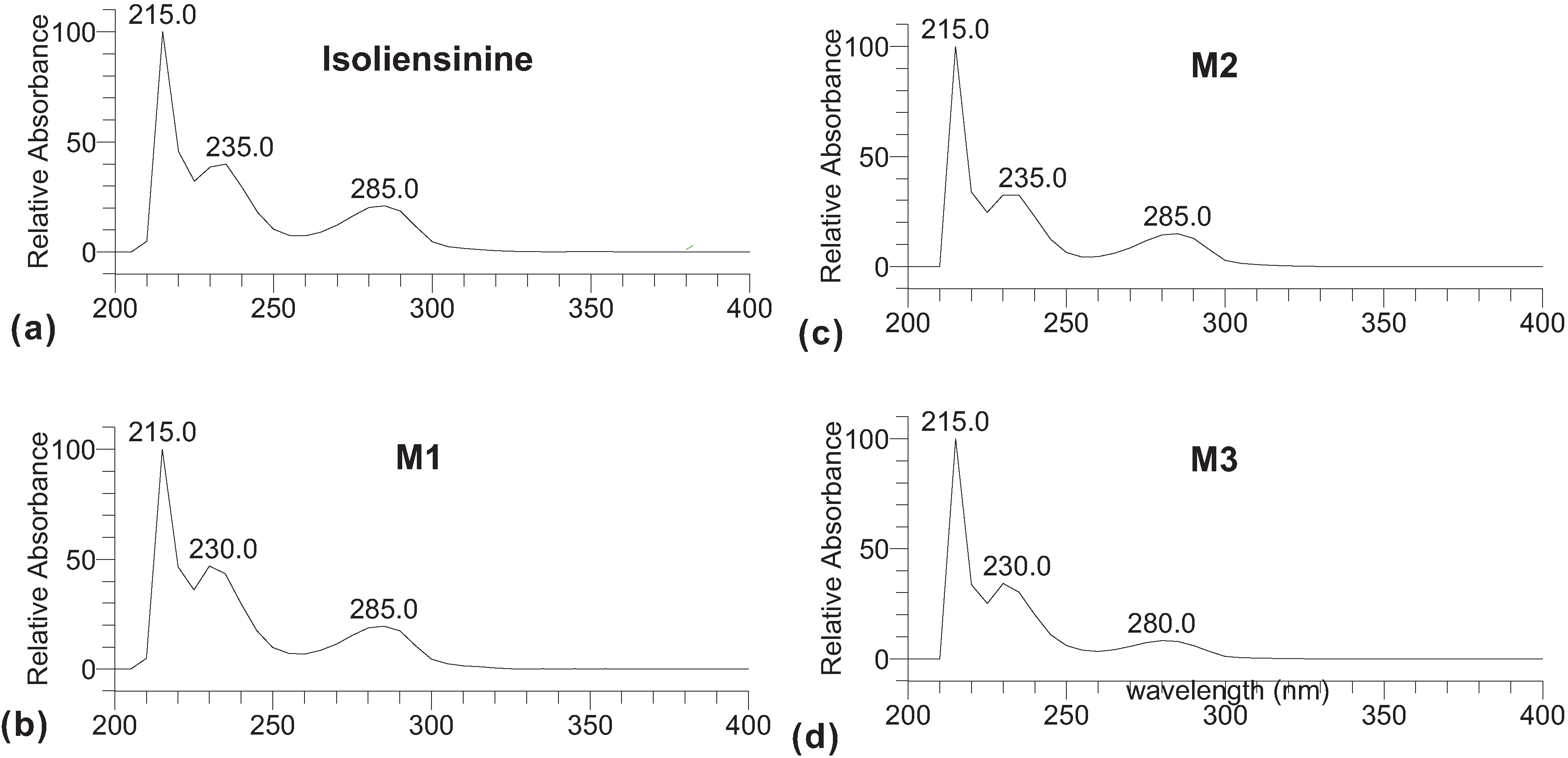
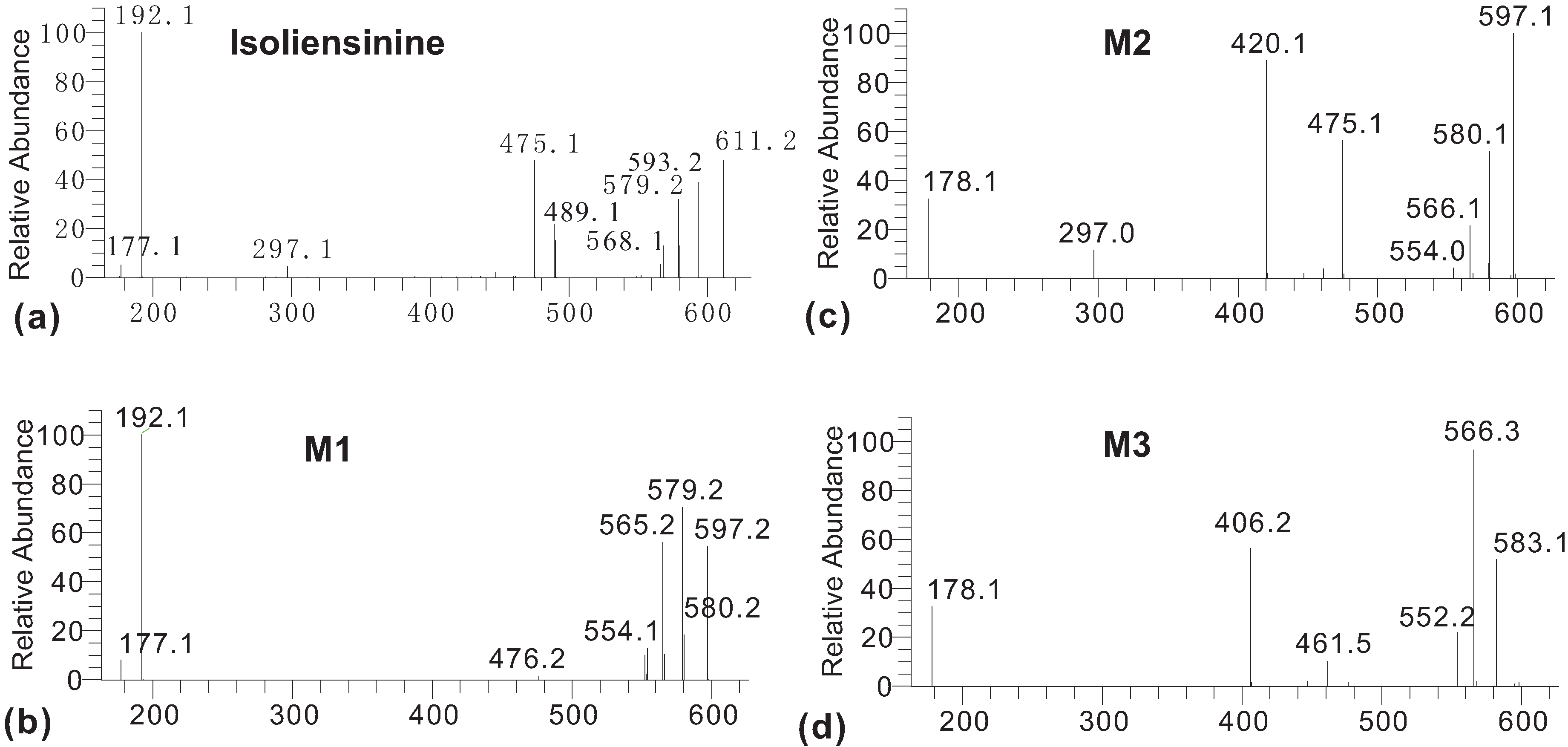
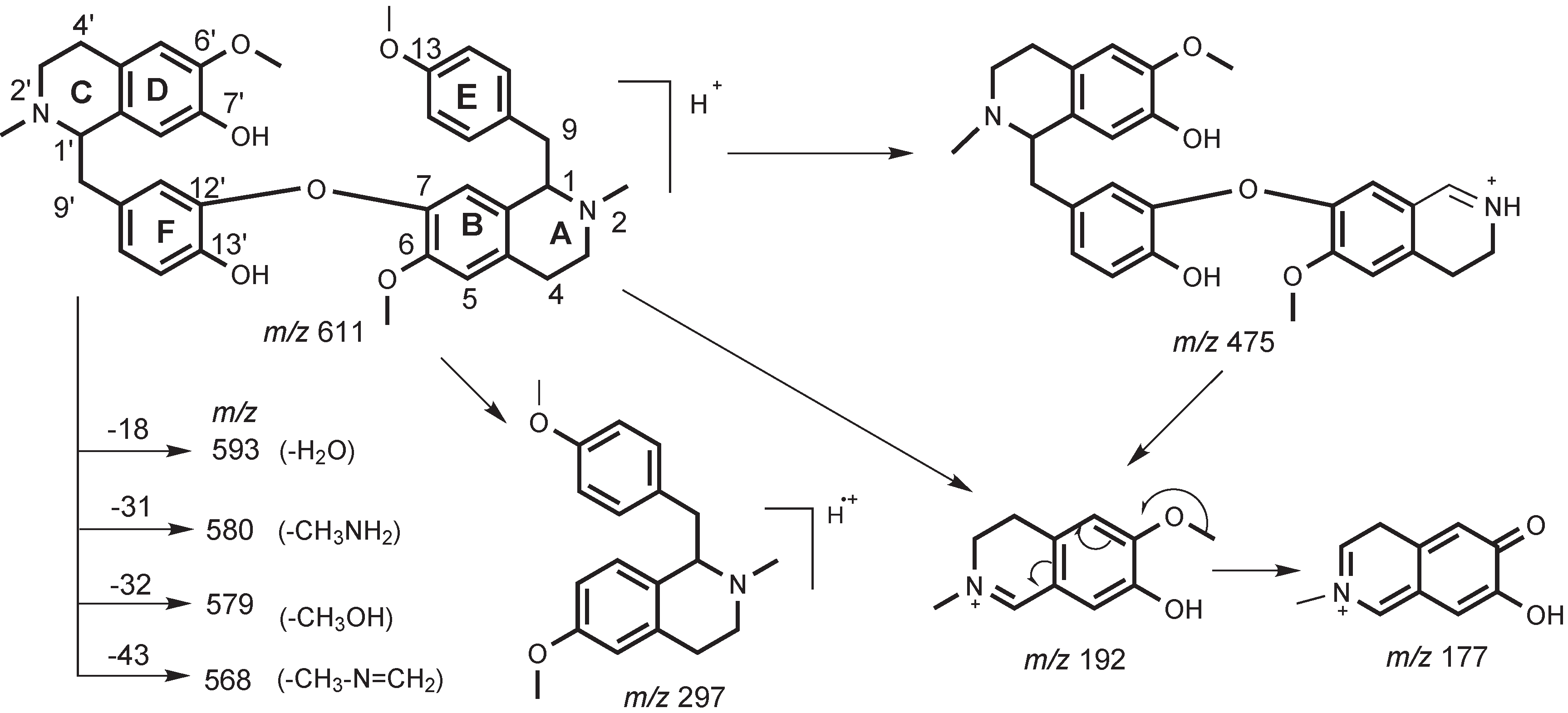
3. Experimental
3.1. Chemicals and Reagents
3.2. Preparation of in Vitro Microsomal Metabolites of Isoliensinine
3.3. Characterization of Metabolites by HPLC-ESI-MS/MS with Data-Dependent Mode
4. Conclusions
Acknowledgments
References
- Schiff, P.L., Jr. Bisbenzylisoquinoline alkaloids. J. Nat. Prod. 1991, 54, 645–749. [Google Scholar] [CrossRef]
- Guha, K.P.; Mukherjee, B.; Mukherjee, R. Bisbenzylisoquinoline Alkaloids—A Review. J. Nat. Prod. 1979, 42, 1–84. [Google Scholar] [CrossRef]
- Ni, X. Studies on the classification of Chinese lotus cultivars. Acta Hortic. Sin. 1983, 10, 207–210. [Google Scholar]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)—phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar]
- Qian, J.Q. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol. Sin. 2002, 23, 1086–1092. [Google Scholar]
- Ai, X.-H.; Tang, X.-Q.; Liu, Y.-P.; Liu, H.-Q.; Dong, L. Effect of neferine on adriamycin-resistance of thermotolerant hepatocarcinoma cell line HepG2/thermotolerance. Ai Zheng 2007, 26, 357–360. [Google Scholar]
- Xiong, Y.Q.; Zeng, F.D. Effect of neferine on toxicodynamics of dichlorvos for inhibiting rabbit cholinesterase. Acta Pharmacol. Sin. 2003, 24, 332–336. [Google Scholar]
- Wang, J.L.; Nong, Y.; Jing, M.X. Effects of liensinine on haemodynamics in rats and the physiologic properties of isolated rabbit atria. Yao Xue Xue Bao 1992, 27, 881–885. [Google Scholar]
- Chen, W.Z.; Ling, S.J.; Ting, K.S. Hypotensive Action of Liensinine and Its Two Derivatives. Yao Xue Xue Bao 1962, 13, 277–280. [Google Scholar]
- Xiao, X.B.; Xie, Z.X.; Chen, J.; Qin, Q.; Zhu, Y. Effect of neferine on the chemotherapic sensitivity of STI 571 to K562/A02 cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2005, 30, 558–561. [Google Scholar]
- Fu, L.W.; Deng, Z.A.; Pan, Q.C.; Fan, W. Screening and discovery of novel MDR modifiers from naturally occurring bisbenzylisoquinoline alkaloids. Anticancer Res. 2001, 21, 2273–2280. [Google Scholar]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Sureram, S.; Senadeer, S.P.D.; Hongmanee, P.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2012, 22, 2902–2905. [Google Scholar] [CrossRef]
- Xiao, J.H.; Zhang, J.H.; Chen, H.L.; Feng, X.L.; Wang, J.L. Inhibitory effects of isoliensinine on bleomycin-induced pulmonary fibrosis in mice. Planta Med. 2005, 71, 225–230. [Google Scholar] [CrossRef]
- Xiao, J.H.; Zhang, Y.L.; Feng, X.L.; Wang, J.L.; Qian, J.Q. Effects of isoliensinine on angiotensin II-induced proliferation of porcine coronary arterial smooth muscle cells. J. Asian Nat. Prod. Res. 2006, 8, 209–216. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Cai, H.; Luo, S.; Wang, J. Pharmacokinetics of neferine in rabbits. Chin. J. Hosp. Pharm. 1993, 13, 105–107. [Google Scholar]
- Hu, X.; Zhang, X.; Cai, H.; Luo, S.; Yin, W. Pharmacokinetics of liensinine in rabbits. Zhongguo Zhong Yao Za Zhi 1992, 17, 622–624. [Google Scholar]
- Xu, L.; Wang, S.; Chen, J.; Yao, C. The pharmacokinetic research on liensinien in rats. Shengyang Yaoke Daxue Xuebao 2001, 18, 244–246. [Google Scholar]
- Huang, Y.; Bai, Y.; Zhao, L.; Hu, T.; Hu, B.; Wang, J.; Xiang, J. Pharmacokinetics and metabolism of neferine in rats after a single oral administration. Biopharm. Drug Dispos. 2007, 28, 361–372. [Google Scholar] [CrossRef]
- Jiang, M.; Liang, X.; Xiong, Y. Metabolic characteristics of neferine in the cytochrome P450 of rat liver microsomes. Chin. Pharmacol. Bull. 2006, 22, 739–743. [Google Scholar]
- Zhou, H.; Jiang, H.; Yao, T.; Zeng, S. Fragmentation study on the phenolic alkaloid neferine and its analogues with anti-HIV activities by electrospray ionization tandem mass spectrometry with hydrogen/deuterium exchange and its application for rapid identification of in vitro microsomal metabolites of neferine. Rapid Commun. Mass Spectrom. 2007, 21, 2120–2128. [Google Scholar] [CrossRef]
- Wu, W.N.; Moyer, M.D. API-ionspray MS and MS/MS study on the structural characterization of bisbenzylisoquinoline alkaloids. J. Pharm. Biomed. Anal. 2004, 34, 53–66. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, H.; Wang, J. Spectroscopic elucidation of liensinine. Zhong Cao Yao 1998, 29, 364–367. [Google Scholar]
- Wu, W.-N.; McKown, L.A. The in vitro metabolism of thalicarpine, an aporphine–benzyltetrahydroisoquinoline alkaloid, in the rat: API-MS/MS identification of thalicarpine and metabolites. J. Pharm. Biomed. Anal. 2002, 30, 141–150. [Google Scholar] [CrossRef]
- Itoh, A.; Saitoh, T.; Tani, K.; Uchigaki, M.; Sugimoto, Y.; Yamada, J.; Nakajima, H.; Ohshiro, H.; Sun, S.; Tanahashi, T. Bisbenzylisoquinoline alkaloids from Nelumbo nucifera. Chem. Pharm. Bull. (Tokyo) 2011, 59, 947–951. [Google Scholar] [CrossRef]
- Hollenberg, P.F. Mechanisms of cytochrome P450 and peroxidase-catalyzed xenobiotic metabolism. FASEB J. 1992, 6, 686–694. [Google Scholar]
- Chen, S.; Liu, L.; Yang, Y.; Dai, Z.; Zeng, F. Metabolism of Dauricine and identification of its main metabolites. J. Tongji Med. Univ. 2000, 20, 253–256. [Google Scholar] [CrossRef]
- Wu, S.H.; Sun, C.R.; Cao, X.J.; Zhou, H.; Zhang, H.; Pan, Y.J. Preparative counter-current chromatography isolation of liensinine and its analogues from embryo of the seed of Nelumbo nucifera GAERTN. using upright coil planet centrifuge with four multilayer coils connected in series. J. Chromatogr. A 2004, 1041, 153–162. [Google Scholar] [CrossRef]
- Gibson, G.; Skett, P. Introduction to Drug Metabolism; Chapman and Hall Ltd.: London, UK & New York, NY, USA, 1986; p. 240. [Google Scholar]
- Sample Availability: Contact the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, H.; Li, L.; Jiang, H.; Zeng, S. Identification of Three New N-Demethylated and O-Demethyled Bisbenzylisoquinoline Alkaloid Metabolites of Isoliensinine from Dog Hepatic Microsomes. Molecules 2012, 17, 11712-11720. https://doi.org/10.3390/molecules171011712
Zhou H, Li L, Jiang H, Zeng S. Identification of Three New N-Demethylated and O-Demethyled Bisbenzylisoquinoline Alkaloid Metabolites of Isoliensinine from Dog Hepatic Microsomes. Molecules. 2012; 17(10):11712-11720. https://doi.org/10.3390/molecules171011712
Chicago/Turabian StyleZhou, Hui, Liping Li, Huidi Jiang, and Su Zeng. 2012. "Identification of Three New N-Demethylated and O-Demethyled Bisbenzylisoquinoline Alkaloid Metabolites of Isoliensinine from Dog Hepatic Microsomes" Molecules 17, no. 10: 11712-11720. https://doi.org/10.3390/molecules171011712



