Hesperidin Prevents Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
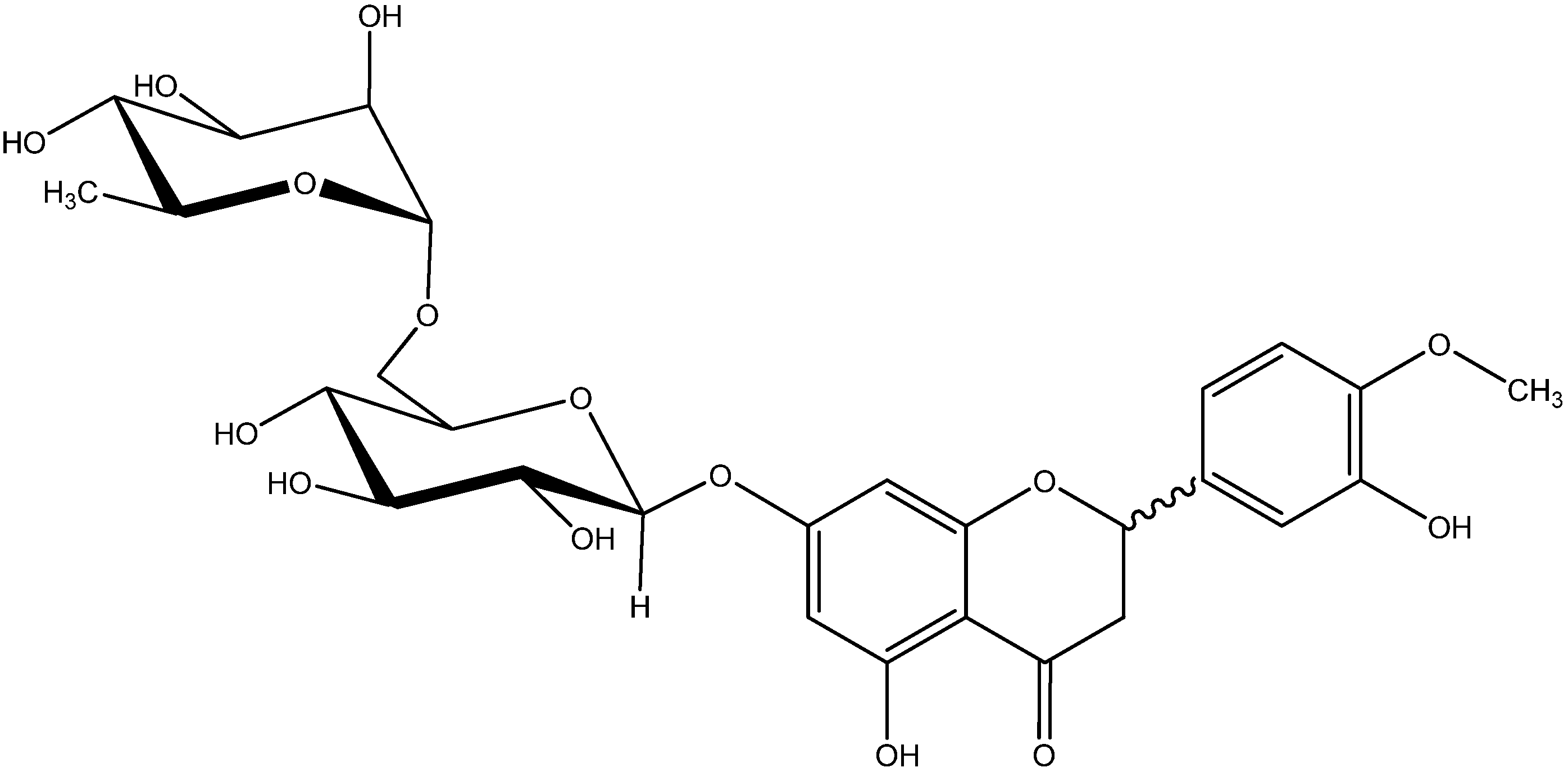
2. Results and Discussion
2.1. Body Weight and Glycemic Parameters
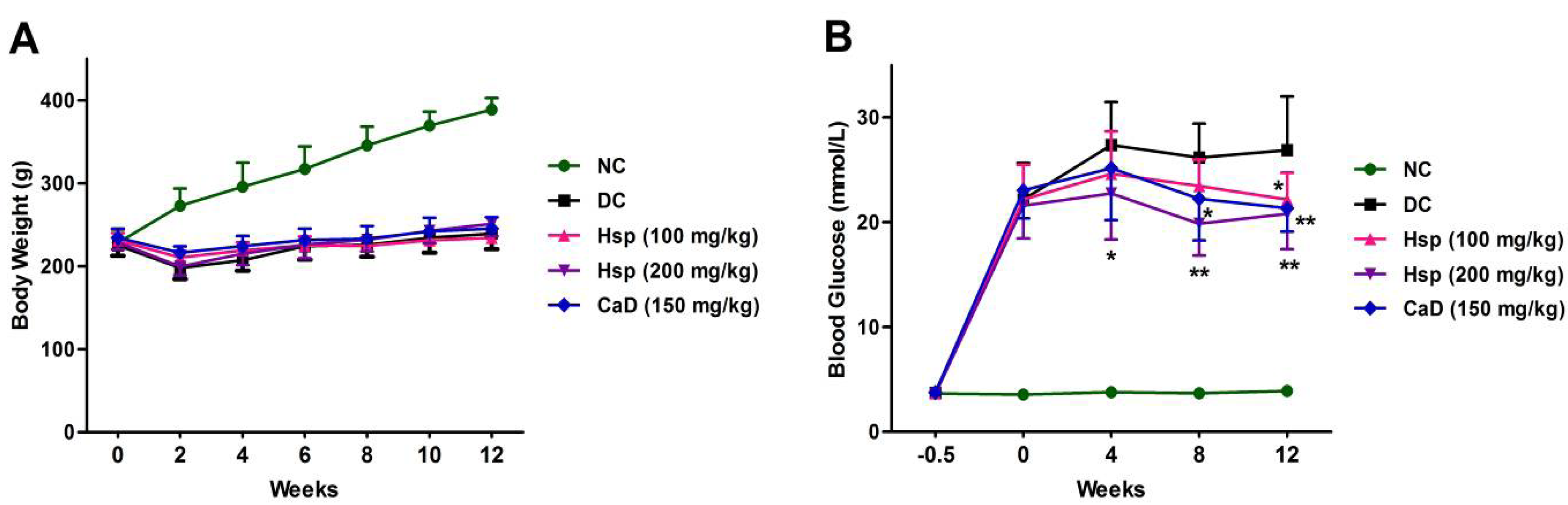
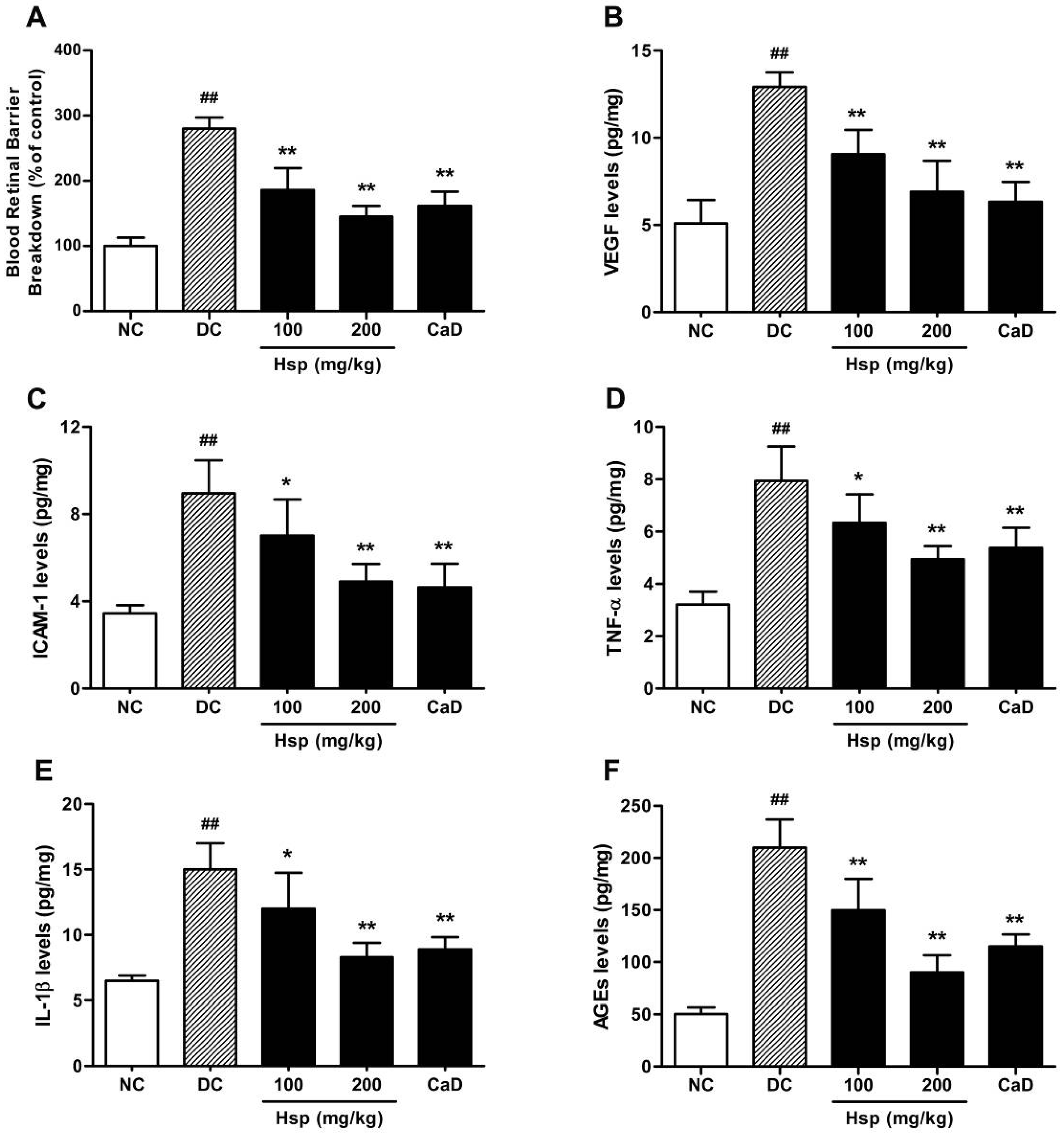
2.2. BRB Breakdown
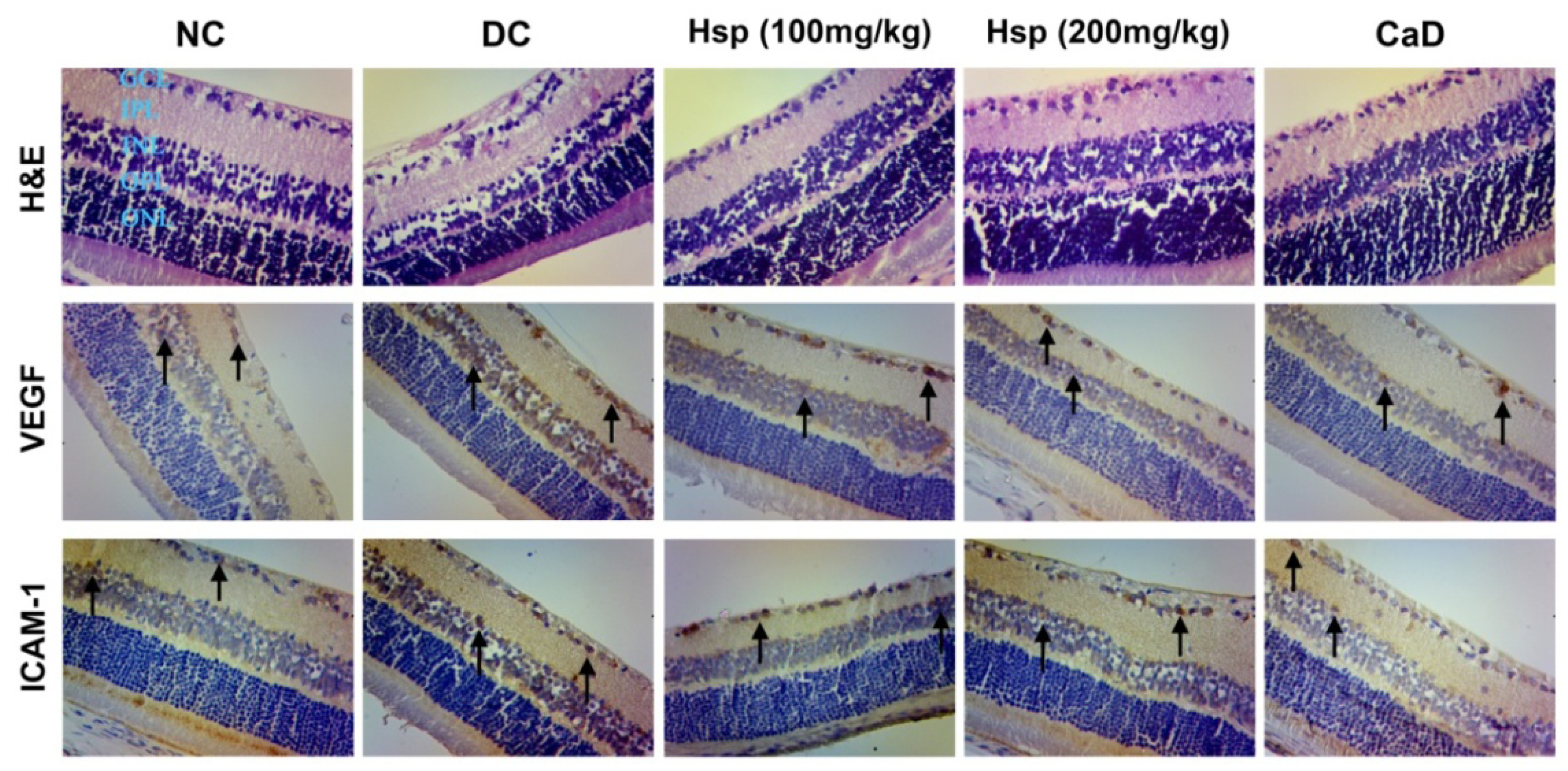
2.3. Retinal VEGF, ICAM-1, TNF-α, IL-1β, and AGEs Levels
2.4. Aldose Reductase (AR) Activity
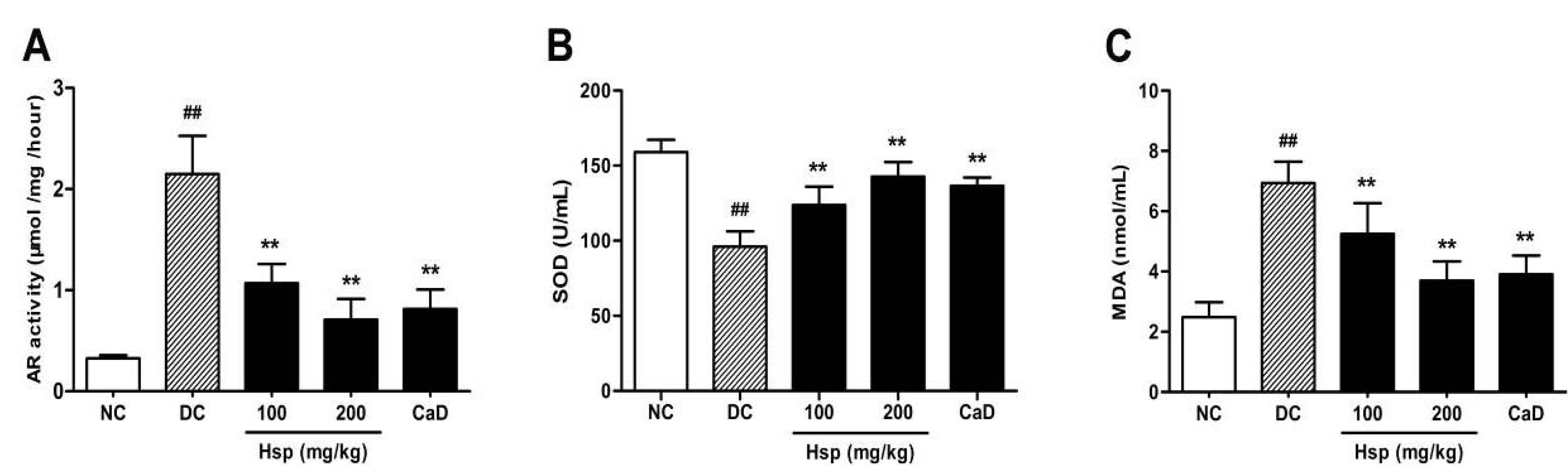
2.5. SOD Activity and MDA Levels in Blood
2.6. Histopathological and Immunohistochemical Studies

3. Experimental
3.1. Chemicals and Drugs
3.2. Animals
3.3. Induction of Diabetes
3.4. Body Weight and Blood Glucose
3.5. Measurement of BRB Breakdown

3.6. Retinal VEGF, ICAM-1, TNF-α, IL-1β and AGEs Levels
3.7. Measurements of SOD and MDA Levels in Serum
3.8. Aldose Reductase Enzyme (AR) Activity
3.9. Histopathological and Immunohistochemical Studies
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Conflict of Interest
References
- Javitt, J.C.; Aiello, L.P.; Chiang, Y.; Ferris, F.L., III; Canner, J.K.; Greenfield, S. Preventive eye care in people with diabetes is cost-saving to the federal government. Implications for health-care reform. Diabetes Care 1994, 8, 909–917. [Google Scholar]
- Moss, S.E.; Klein, R.; Klein, B.E. The 14-year incidence of visual loss in a diabetic population. Ophthalmology 1998, 105, 998–1003. [Google Scholar] [CrossRef]
- Hazin, R.; Colyer, M.; Lum, F.; Barazi, M.K. Revisiting diabetes 2000: Challenges in establishing nationwide diabetic retinopathy prevention programs. Am. J. Ophthalmol. 2011, 152, 723–729. [Google Scholar] [CrossRef]
- Frank, R.N. Vascular endothelial growth factor—Its role in retinal vascular proliferation. N. Engl. J. Med. 1994, 331, 1519–1520. [Google Scholar] [CrossRef]
- Adler, A.I.; Stevens, R.J.; Neil, A.; Stratton, I.M.; Boulton, A.J.; Holman, R.R. UKPDS 59: Hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care 2002, 5, 894–899. [Google Scholar]
- Arden, G.B.; Sivaprasad, S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr. Diabetes Rev. 2011, 7, 291–304. [Google Scholar]
- Chung, S.S.; Chung, S.K. Aldose reductase in diabetic microvascular complications. Curr. Drug Targets 2005, 6, 475–486. [Google Scholar] [CrossRef]
- Curtis, T.M.; Scholfield, C.N. The role of lipids and protein kinase Cs in the pathogenesis of diabetic retinopahy. Diabetes Metab. Res. Rev. 2004, 20, 28–43. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 1, 3–21. [Google Scholar] [CrossRef]
- Gustavsson, C.; Agardh, E.; Bengtsson, B.; Agardh, C. TNF-α is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J. Diabetes Complicat. 2008, 22, 309–316. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Mitsiades, N.; Kirchhof, B.; Koizumi, K.; Dohmen, S.; Adamis, A.P. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-α suppression. FASEB J. 2002, 16, 438–440. [Google Scholar]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of leuostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. USA 1996, 96, 10836–10841. [Google Scholar]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar]
- Kusari, J.; Zhou, S.X.; Padillo, E.; Clarke, K.G.; Gil, D.W. Inhibition of vitreoretinal VEGF elevation and blood-retinal barrier breakdown in streptozotocin-induced diabetic rats by brimonidine. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1044–1051. [Google Scholar] [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Moromizato, Y.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am. J. Pathol. 2000, 156, 1733–1739. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat 2012, in press. [Google Scholar]
- Kim, S.W.; Kim, C.E.; Kim, M.H. Flavonoids inhibit high glucose-induced up-regulation of ICAM-1 via the p38 MAPK pathway in human vein endothelial cells. Biochem. Biophys. Res. Commun. 2011, 415, 602–607. [Google Scholar] [CrossRef]
- Liu, X.R.; Zhang, Y.; Lin, Z.Q. Advances in studies on the biological activities of hesperidin and hesperetin. Chin. J. New Drugs 2011, 20, 329–333. [Google Scholar]
- Chew, E.Y.; Klein, M.L.; Ferris, F.L.; Remaley, N.A.; Murphy, R.P.; Chantry, K.; Hoogwerf, B.J.; Miller, D. Association of elevated serum lipid with retinal hard exudate in diabetic retinopathy. Arch. Ophthalmol. 1996, 114, 1079–1084. [Google Scholar] [CrossRef]
- Jain, S.K.; McVie, R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in type 1 diabetic patients. Diabetes 1999, 48, 1850–1855. [Google Scholar] [CrossRef]
- Kern, T.S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 95–103. [Google Scholar]
- Agrawal, S.S.; Naqvi, S.; Gupta, S.K.; Srivastava, S. Prevention and management of diabetic retinopathy in STZ diabetic rats by Tinospora cordifolia and its molecular mechanisms. Food Chem. Toxicol. 2012, 50, 3126–3132. [Google Scholar] [CrossRef]
- Klaassen, I.; Hughes, J.M.; Vogels, I.M.; Schalkwijk, C.G.; van Noorden, C.J.; Schlingemann, R.O. Altered expression of genes related to blood–retina barrier disruption in streptozotocin-induced diabetes. Exp. Eye Res. 2009, 1, 4–15. [Google Scholar]
- Leal, E.C.; Martins, J.; Voabil, P.; Liberal, J.; Chiavaroli, C.; Bauer, J.; Cunha-vaz, J.; Ambrósio, A.F. Calcium Dobesilate Inhibits the Alterations in Tight Junction Proteins and Leukocyte Adhesion to Retinal Endothelial Cells Induced by Diabetes. Diabetes 2010, 59, 2637–2645. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Aiello, L.P.; Wong, J.S. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int. 2000, 58, 113–119. [Google Scholar] [CrossRef]
- Detmar, M.; Brown, L.F.; Schon, M.P.; Elicker, B.M.; Velasco, P.; Richard, L.; Fukumura, D.; Monsky, W.; Claffey, K.P.; Jain, R.K. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J. Invest. Dermatol. 1998, 111, 1–6. [Google Scholar] [CrossRef]
- Adamis, A.P. Is diabetic retinopathy an inflammatory disease? Br.J. Ophthalmol. 2002, 86, 363–365. [Google Scholar] [CrossRef]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Ahmed, E.; Carrasquillo, K.G.; Amano, S.; Hida, T.; Oguchi, Y.; Adamis, A.P. VEGF164 is proinflammatory in the diabetic retina. Invest. Ophthalmol.Vis. Sci. 2003, 5, 2155–2162. [Google Scholar]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- McLend, D.S.; Lefer, D.J.; Merges, C.; Lutty, G.A. Enhanced expression of intracellular adhesion molecule-1 and pselectin in the diabetic human retina and choroids. Am. J. Pathol. 1995, 147, 642–653. [Google Scholar]
- Luo, D.W.; Fan, Y.; Xu, X. The effects of aminoguanidine on retinopathy in STZ-induced diabetic rats. Bioorg. Med. Chem. Lett. 2012, 13, 4386–4390. [Google Scholar]
- Drel, V.R.; Pacher, P.; Ali, T.K.; Shin, J.; Julius, U.; EI-Remessy, A.B.; Obrosova, I.G. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int. J. Mol. Med. 2008, 6, 667–676. [Google Scholar]
- Yülek, F.; Or, M.; Özoğul, C.; Isik, A.C.; Ari, N.; Stefek, M.; Bauer, V.; Karasu, C. Effects of Stobadine and Vitamin E in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Arch. Med. Res 2007, 38, 503–511. [Google Scholar] [CrossRef]
- Kamper, M.; Tsimpoukidi, O.; Chataigeorgiou, A.; Lymberi, M.; Kamper, E.F. The antioxidant effect of angiotensin II receptor blocker, Iosatan, in streptozotocin-induced diabetic rats. Transl. Res. 2010, 1, 26–36. [Google Scholar]
- Kowluru, R.A.; Kowluru, V.; Ho, Y.S.; Xiong, Y. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic. Biol. Med. 2006, 41, 1191–1196. [Google Scholar] [CrossRef]
- Park, J.W.; Park, S.J.; Park, S.H.; Kim, K.Y.; Chung, J.W.; Chun, M.H.; Oh, S.J. Up-regulated expression of neuronal nitric oxide synthase in experimental diabetic retina. Neurobiol. Dis. 2006, 1, 43–49. [Google Scholar]
- Zhang, Q.; Guy, K.; Pagadala, J.; Jiang, Y.; Walker, R.J.; Liu, L.; Soderland, C.; Kern, T.S.; Ferry, R., Jr.; He, H.; et al. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 levels. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3004–3013. [Google Scholar]
- Bucolo, C.; Ward, K.W.; Mazzon, E.; Cuzzocrea, S.; Drago, F. Protective effects of a coumarin derivative in diabetic rats. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3846–3852. [Google Scholar] [CrossRef]
- Li, D.; Mitsuhashi, S.; Ubukata, M. Protective effects of hesperidin derivatives and their stereoisomers against advanced glycation end-products formation. Pham. Biol 2012. [Google Scholar] [CrossRef]
- Brand, W.; Shao, J.; Hoek-van Den Hil, E.F.; van Elk, K.N.; Spenkelink, B.; de Haan, L.H.; Rein, M.J.; Dionisi, F.; Williamson, G.; van Bladeren, P.J.; et al. Stereoselective Conjugation, Transport and Bioactivity of S- and R-Hesperetin Enantiomers in Vitro. J. Agric. Food Chem. 2010, 58, 6119–6125. [Google Scholar]
- Srirangam, R.; Hippalgaonkar, K.; Avula, B.; Khan, I.A.; Majumdar, S. Evaluation of the Intravenous and Topical Routes for Ocular Delivery of Hesperidin and Hesperetin. J. Ocul. Pharmacol. Ther. 2012. [Google Scholar] [CrossRef]
- Sample Availability: Samples of hesperidin are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, X.; Liao, S.; Mi, H.; Guo, C.; Qi, D.; Li, F.; Zhang, C.; Yang, Z. Hesperidin Prevents Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats. Molecules 2012, 17, 12868-12881. https://doi.org/10.3390/molecules171112868
Shi X, Liao S, Mi H, Guo C, Qi D, Li F, Zhang C, Yang Z. Hesperidin Prevents Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats. Molecules. 2012; 17(11):12868-12881. https://doi.org/10.3390/molecules171112868
Chicago/Turabian StyleShi, Xiupu, Sha Liao, Huijuan Mi, Changrun Guo, Dongli Qi, Fei Li, Chunfeng Zhang, and Zhonglin Yang. 2012. "Hesperidin Prevents Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats" Molecules 17, no. 11: 12868-12881. https://doi.org/10.3390/molecules171112868



