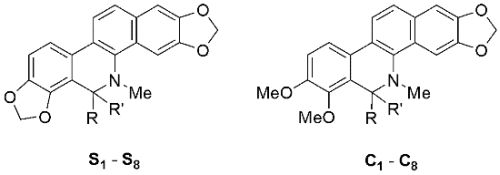
In Vitro Antifungal Activity of Sanguinarine and Chelerythrine Derivatives against Phytopathogenic Fungi
Abstract
:1. Introduction
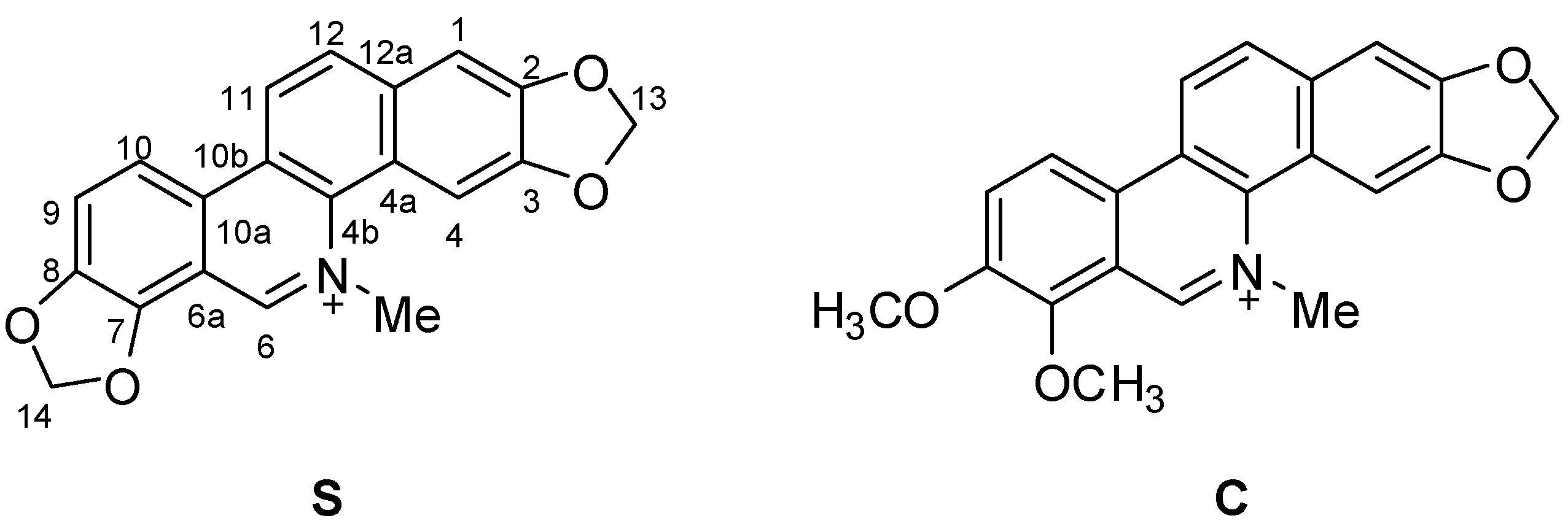
2. Results and Discussion
2.1. Chemistry
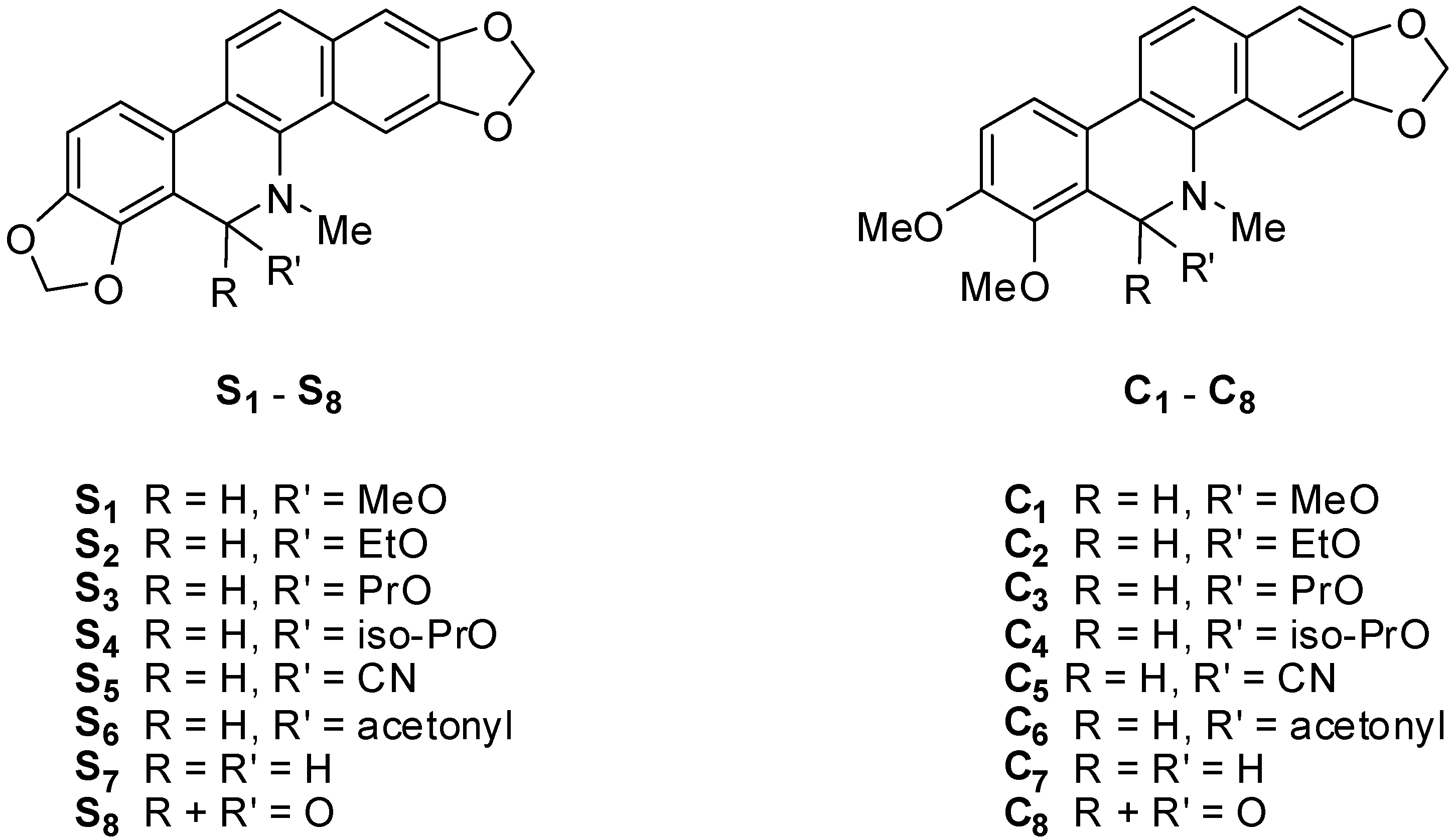
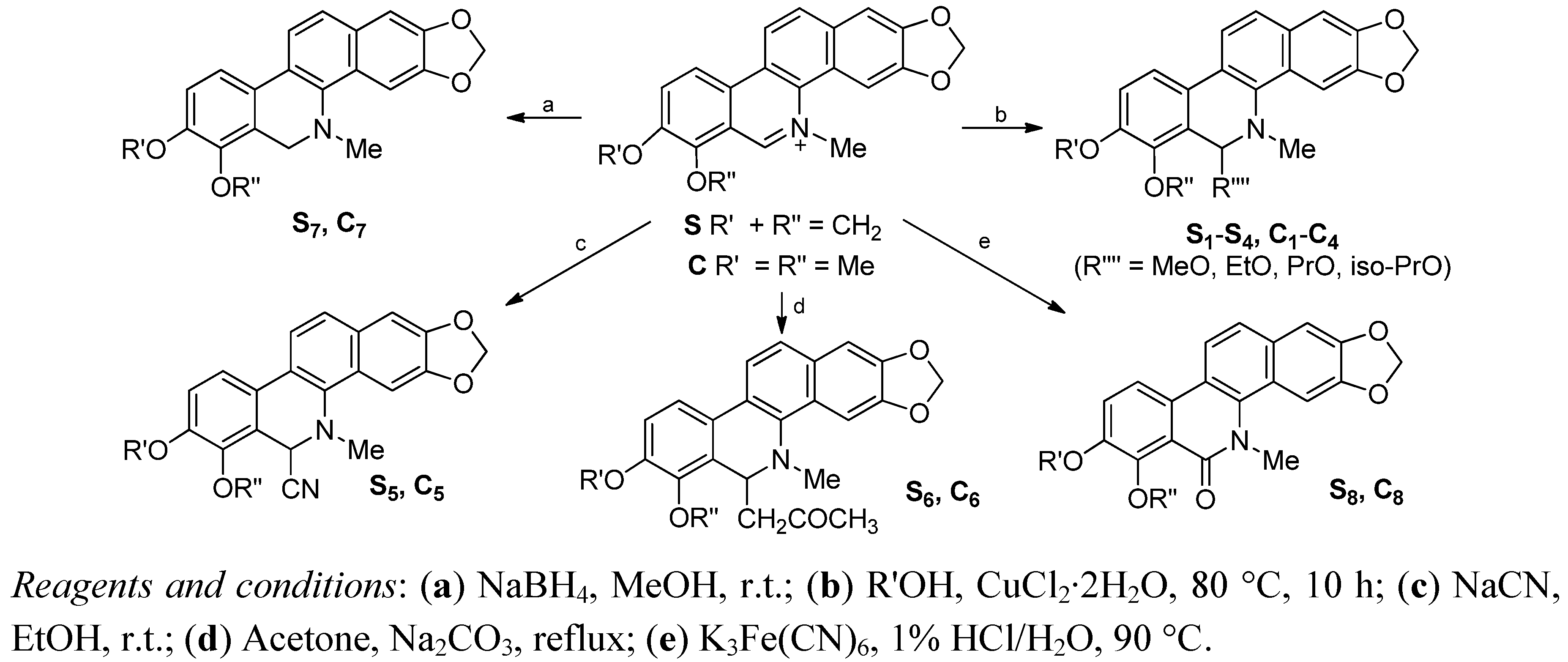
2.2. Antifungal Activity
2.2.1. Screening of Antifungal Activity in Vitro
| Compd. | Seven Tested Phytopathogenic Fungi * | ||||||
|---|---|---|---|---|---|---|---|
| C.L. | V.M. | F.S. | F.O.N. | F.O.V. | P. O. | A.A. | |
| S | 78.6 ± 3.4DE ** | 84.8 ± 0.8BC | 81.4 ± 1.0BC | 72.1 ± 3.1E | 82.0 ± 2.2D | 60.4 ± 2.1D | 71.2 ± 0.0C |
| S1 | 80.8 ± 2.0BCDE | 88.1 ± 0.4AB | 83.8 ± 2.6BC | 86.6 ± 1.5C | 84.0 ± 0.0CD | 70.8 ± 2.1C | 74.6 ± 0.9C |
| S2 | 87.5 ± 2.8A | 85.8 ± 5.9BC | 85.4 ± 2.6B | 69.7 ± 1.7E | 85.6 ± 1.6CD | 70.1 ± 3.2C | 70.0 ± 2.3C |
| S3 | 86.1 ± 0.8AB | 93.2 ± 0.6A | 86.9 ± 4.6AB | 87.6 ± 2.3C | 87.2 ± 1.6C | 69.4 ± 1.2C | 73.6 ± 0.9C |
| S4 | 78.1 ± 1.5DE | 84.6 ± 1.8BC | 82.0 ± 2.1BC | 79.6 ± 5.4D | 84.5 ± 1.0CD | 61.1 ± 1.2D | 64.9 ± 1.5D |
| S5 | 79.7 ± 0.8CDE | 80.1 ± 0.4C | 73.3 ± 0.0D | 63.2 ± 0.0F | 71.6 ± 0.7E | 47.0 ± 0.6E | 44.9 ± 1.6E |
| S6 | 19.1 ± 2.0HI | −4.0 ± 3.5FG | −0.1 ± 3.2F | −0.5 ± 0.9K | −4.4 ± 0.9J | 9.7 ± 1.2HI | 16.2 ± 1.5H |
| S7 | 33.0 ± 1.3F | −8.3 ± 1.7H | 7.5 ± 1.9E | 2.0 ± 3.4JK | −0.1 ± 1.8HI | 20.8 ± 0.0G | 21.2 ± 0.9G |
| S8 | 18.0 ± 1.3I | 3.4 ± 0.7DE | 6.3 ± 0.7E | 0.5 ± 0.9JK | 2.6 ± 3.9H | 5.8 ± 1.2IJ | 11.0 ± 1.5I |
| C | 81.5 ± 2.2BCDE | 88.5 ± 2.0AB | 87.3 ± 0.2AB | 85.6 ± 0.9C | 94.2 ± 0.0B | 74.3 ± 1.2BC | 83.7 ± 1.8B |
| C1 | 79.9 ± 3.5CDE | 93.0 ± 1.7A | 92.4 ± 0.4A | 94.0 ± 0.0B | 100.0 ± 0.0A | 77.1 ± 2.1AB | 85.8 ± 0.9AB |
| C2 | 76.3 ± 0.8E | 86.9 ± 0.4AB | 83.4 ± 0.2BC | 100.0 ± 0.0A | 95.7 ± 3.7AB | 79.9 ± 1.2AB | 86.8 ± 0.9AB |
| C3 | 85.7 ± 5.1ABC | 89.8 ± 2.1AB | 86.4 ± 6.4AB | 94.0 ± 0.0B | 100.0 ± 0.0A | 81.9 ± 2.4A | 87.3 ± 0.9AB |
| C4 | 82.6 ± 4.0ABCD | 83.5 ± 0.8BC | 82.2 ± 0.9BC | 90.5 ± 0.9BC | 92.0 ± 0.0B | 83.0 ± 0.6A | 88.8 ± 0.9A |
| C5 | 80.8 ± 0.3BCDE | 84.4 ± 0.4BC | 78.7 ± 0.4CD | 40.2 ± 3.4G | 69.1 ± 1.1E | 48.4 ± 1.1E | 62.5 ± 0.8D |
| C6 | 5.7 ± 3.4J | −1.4 ± 2.8EF | 8.6 ± 3.9E | 5.0 ± 0.9IJ | −3.3 ± 3.3IJ | 1.4 ± 2.4J | 5.5 ± 1.5J |
| C7 | 18.0 ± 1.3I | 5.1 ± 6.9D | 10.5 ± 3.2E | 13.4 ± 0.0H | 12.7 ± 2.4F | 28.9 ± 5.9F | 47.9 ± 5.9E |
| C8 | 24.0 ± 2.0GH | −5.2 ± 2.4FG | 10.5 ± 1.3E | 9.5 ± 4.8HI | 6.8 ± 0.9G | 11.9 ± 1.3H | 24.7 ± 1.8G |
| TBZ *** | 27.1 ± 1.5G | 88.4 ± 0.8AB | 83.9 ± 3.5BC | 100.0 ± 0.0A | 100.0 ± 0.0A | 15.3 ± 6.7GH | 30.6 ± 4.0F |
2.2.2. Antifungal Toxicity
| Fungus | Compd. | Toxicity regression equation * | R2 | EC50 value | CI 95% ** (µg/mL) | CA *** | |
|---|---|---|---|---|---|---|---|
| (µg/mL) | (µM) | ||||||
| C.L. | S | y = 0.5758x − 0.3905 | 0.9628 | 35.20 | 76.6 | 35.13–35.27 | 16.4 |
| S1 | y = 0.5452x − 0.2960 | 0.9848 | 28.84 | 79.4 | 28.81–28.85 | 18.9 | |
| C | y = 0.3413x + 0.0944 | 0.9621 | 15.43 | 32.5 | 15.21–15.65 | 22.1 | |
| C1 | y = 0.3635x + 0.0808 | 0.9914 | 14.23 | 37.5 | 14.18–14.28 | 25.5 | |
| V.M. | S | y = 0.6317x − 0.3751 | 0.9901 | 24.28 | 52.9 | 24.24–24.32 | 26.0 |
| S1 | y = 0.5275x − 0.2329 | 0.9860 | 24.51 | 67.5 | 23.97–25.05 | 21.5 | |
| C | y = 0.6918x − 0.6038 | 0.9505 | 39.40 | 82.9 | 39.30–39.50 | 17.6 | |
| C1 | y = 0.6420x − 0.4423 | 0.9904 | 29.36 | 77.4 | 29.34–29.38 | 21.9 | |
| F.S. | S | y = 0.7415x − 0.6922 | 0.9717 | 40.53 | 88.3 | 40.46–40.60 | 18.3 |
| S1 | y = 0.5949x − 0.4189 | 0.957 | 35.04 | 96.4 | 34.95–35.13 | 17.0 | |
| C | y = 0.5005x − 0.2123 | 0.9600 | 26.50 | 55.8 | 26.41–26.59 | 18.9 | |
| C1 | y = 0.5949x − 0.3540 | 0.9925 | 27.26 | 71.8 | 27.24–27.28 | 21.8 | |
| F.O.N. | S | y = 0.5139x − 0.3676 | 0.9768 | 48.79 | 106.2 | 48.73–48.85 | 10.9 |
| S1 | y = 0.5487x − 0.3317 | 0.9705 | 32.79 | 90.2 | 32.73–32.85 | 16.7 | |
| C | y = 0.6186x − 0.4076 | 0.9891 | 29.32 | 61.7 | 29.30–29.34 | 21.1 | |
| C1 | y = 0.6629x − 0.4624 | 0.9836 | 28.30 | 74.6 | 28.26–28.34 | 23.4 | |
| F.O.V. | S | y = 0.6007x − 0.4137 | 0.9735 | 33.20 | 72.3 | 33.14–33.26 | 18.1 |
| S1 | y = 0.6320x − 0.4735 | 0.9826 | 34.70 | 95.5 | 34.66–34.74 | 18.2 | |
| C | y = 1.0309x − 1.2677 | 0.9600 | 51.84 | 109.1 | 51.80–51.88 | 19.9 | |
| C1 | y = 0.6303x − 0.4308 | 0.9684 | 29.98 | 79.0 | 29.91–30.06 | 21.0 | |
| P.O. | S | y = 0.3182x − 0.1386 | 0.9687 | 101.6 | 221.2 | 101.2–102.00 | 3.1 |
| S1 | y = 0.4238x − 0.3413 | 0.9914 | 96.63 | 265.9 | 96.61-96.63 | 4.4 | |
| C | y = 0.7447x − 0.7550 | 0.9727 | 48.44 | 101.9 | 48.41–48.47 | 15.4 | |
| C1 | y = 0.7178x − 0.6779 | 0.9843 | 43.75 | 115.3 | 43.72–43.78 | 16.4 | |
| A.A. | S | y = 0.7921x − 0.8663 | 0.9608 | 53.08 | 115.6 | 53.03–53.13 | 14.9 |
| S1 | y = 0.5176x − 0.3453 | 0.9193 | 43.47 | 119.6 | 43.10–43.84 | 11.9 | |
| C | y = 0.9270x − 1.0756 | 0.9572 | 50.08 | 105.4 | 50.03–50.13 | 18.5 | |
| C1 | y = 0.6218x − 0.4104 | 0.9659 | 29.11 | 76.7 | 29.04–29.18 | 21.4 | |
2.3. Structure-Activity Relationship
3. Experimental
3.1. General
3.2. Synthesis of S1–S8 and C1–C8
3.3. Screening of Antifungal Activity in Vitro
3.4. Toxicity Assays
3.5. Statistic Analysis
4. Conclusions
Acknowledgments
References
- Wedge, D.E.; Camper, N.D. Biologically Active Natural Products. In Agrochemicals and Pharmaceuticals; Cutler, H.G., Cutler, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–15. [Google Scholar]
- Krane, B.D.; Fagbule, M.O.; Shamma, M.; Gözler, B. The benzophenanthridine alkaloids. J. Nat. Prod. 1984, 47, 1–43. [Google Scholar] [CrossRef]
- Šimánek, V. Benzophenanthridine Alkaloids. In The Alkaloids; Brossi, A., Ed.; Academic Press: New York, NY, USA, 1985; Volume 26, pp. 185–240. [Google Scholar]
- Ahsan, H.; Reagan-Shaw, S.; Breur, J.; Ahmad, N. Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins. Cancer Lett. 2007, 249, 198–208. [Google Scholar] [CrossRef]
- Jang, B.C.; Park, J.G.; Song, D.K.; Baek, W.K.; Yoo, S.K.; Jung, K.H.; Park, G.Y.; Lee, T.Y.; Suh, S.I. Sanguinarine induces apoptosis in A549 human lung cancer cells primarily via cellular glutathione depletion. Toxicol. In Vitro 2009, 23, 281–287. [Google Scholar] [CrossRef]
- Godowski, K.C. Antimicrobial action of sanguinarine. J. Clin. Dent. 1989, 1, 96–101. [Google Scholar]
- Navarro, V.; Villarreal, M.L.; Rojas, G.; Lozoya, X. Antimicrobial evaluation of some plants used in Mexican traditional medicine for the treatment of infectious diseases. J. Ethnopharmacol. 1996, 53, 143–147. [Google Scholar] [CrossRef]
- Zuo, G.Y.; Meng, F.Y.; Hao, X.Y.; Zhang, Y.L.; Wang, G.C.; Xu, G.L. Antibacterial alkaloids from Chelidonium majus Linn (Papaveraceae) against clinical isolates of methicillin-resistant Staphylococcus aureus. J. Pharm. Pharmaceut. Sci. 2008, 11, 90–94. [Google Scholar]
- Meng, F.Y.; Zuo, G.Y.; Hao, X.Y.; Wang, G.C.; Xiao, H.T.; Zhang, J.Q.; Xu, G.L. Antifungal activity of the benzo[c]phenanthridine alkaloids from Chelidonium majus Linn against resistant clinical yeast isolates. J. Ethnopharmacol. 2009, 125, 494–496. [Google Scholar] [CrossRef]
- Lenfeld, J.; Kroutil, M.; Maršálek, E.; Slavík, J.; Preininger, V.; Šimánek, V. Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonoum majus. Planta Med. 1981, 43, 161–165. [Google Scholar] [CrossRef]
- Colombo, M.L.; Bosisio, E. Pharmacological activities of Chelidonium majus L. (Papaveraceae). Pharmacol. Res. 1996, 33, 127–134. [Google Scholar]
- Tan, G.T.; Pezzuto, J.M.; Kinghorn, A.D.; Hughes, S.H. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J. Nat. Prod. 1991, 54, 143–154. [Google Scholar] [CrossRef]
- Yao, J.Y.; Li, X.L.; Shen, J.Y.; Pan, X.Y.; Hao, G.J.; Xu, Y.; Ying, W.L.; Ru, H.S.; Liu, X.L. Isolation of bioactive components from Chelidonium majus L. with activity against Trichodina sp. Aquaculture 2011, 318, 235–238. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhou, Z.; Jiang, D.X.; Han, J.; Wang, J.F.; Zhao, L.W.; Li, J. In vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim) Fedde against Dactylogyrus intermedius in Carassius auratus. Vet. Parasitol. 2010, 171, 305–313. [Google Scholar] [CrossRef]
- Nyangulu, J.M.; Hargreaves, S.L.; Sharples, S.L.; Mackay, S.P.; Waigh, R.D.; Duval, O.; Mberu, E.K.; Watkins, W.M. Antimalarial benzo[c]phenanthridines. Bioorg. Med. Chem. Lett. 2005, 15, 2007–2010. [Google Scholar] [CrossRef]
- Tsai, I.L.; Wun, M.F.; Teng, C.M.; Ishikawa, T.; Chen, I.S. Anti-platelet aggregation constituents from formosan Toddalia asiatica. Phytochemistry 1998, 48, 1377–1382. [Google Scholar] [CrossRef]
- Eun, J.P.; Koh, G.Y. Suppression of angiogenesis by the plant alkaloid, sanguinarine. Biochem. Biophys. Res. Commun. 2004, 317, 618–624. [Google Scholar] [CrossRef]
- Cho, K.M.; Yoo, I.D.; Kim, W.G. 8-Hydroxy dihydrochelerythrine and 8-hydroxy dihydrosanguinarine with a potent acetylcholinesterase inhibitory activity from Chelidonium majus L. Biol. Pharm. Bull. 2006, 29, 2317–2320. [Google Scholar] [CrossRef]
- Miao, F.; Yang, X.J.; Ma, Y.N.; Zheng, F.; Song, X.P.; Zhou, L. Structural modification of sanguinarine and chelerythrine and their in vitro acaricidal activity against Psoroptes cuniculi. Chem. Pharm. Bull. 2012. [Google Scholar] [CrossRef]
- Greathouse, G.A. Alkaloids from Sanguinaria canadensis and their influence on growth of Phymatotrichum omnivorum. Plant Physiol. 1939, 14, 377–380. [Google Scholar] [CrossRef]
- Presley, J. Growth response of Verticillium albo-atrum to sanguinarine in nutrient agar. Phytopathology 1969, 59, 1968–1969. [Google Scholar]
- Howell, C.R.; Bell, A.A.; Stipanovic, R.D. Virulence to cotton and tolerance to sanguinarine among Verticillium species. Can. J. Microbiol. 1973, 19, 1367–1371. [Google Scholar] [CrossRef]
- Matos, O.C.; Baeta, J.; Silva, M.J.; Pinto Ricardo, C.P. Sensitivity of Fusarium strains to Chelidonium majus L. extracts. J. Ethnopharmacol. 1999, 66, 151–158. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhao, J.; Lu, S.; Wang, J.; Jiang, W.; Ma, Z.; Zhou, L. Isoquinoline alkaloids from Macleaya cordata active against plant microbial pathogens. Nat. Prod. Commun. 2009, 4, 1557–1560. [Google Scholar]
- Newman, S.E.; Roll, M.J.; Harkrader, R.J. A naturally occurring compound for controlling powdery mildew of greenhouse roses. HortScience 1999, 34, 686–689. [Google Scholar]
- Miao, F.; Yang, X.J.; Zhou, L.; Hu, H.J.; Zheng, F.; Ding, X.D.; Sun, D.M.; Zhou, C.D.; Sun, W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011, 25, 863–875. [Google Scholar] [CrossRef]
- Dostál, J.; Slavík, J. Some Aspects of the Chemistry of Quaternary Benzo[c]phenanthridine Alkaloids. In Studies in Natural Products Chemistry, 1st; Atta-ur-Rahman, Ed.; Elsevier Scicence B.V: Oxford, UK, 2002; Volume 27, Part H, pp. 155–184. [Google Scholar]
- Slavík, J.; Hanuš, V.; Slavíková, L. Alkaloids from Stylophorum lasiocarpum (Oliv.) Fedde. Collec. Czech. Chem. Commun. 1991, 56, 1116–1122. [Google Scholar]
- Walterová, D.; Ulrichová, J.; Válka, I.; Vicar, J.; Vavrecková, C.; Táborská, E.; Harjrader, R.J.; Meyer, D.L.; Cerná, H.; Simánek, V. Benzo[c]phenanthridine alkaloids sanguinarine and chelerythrine: Biological activities and dental care applications. Acta Univ. Palacki. Olomuc. Fac. Med. 1995, 139, 7–16. [Google Scholar]
- Zhang, J.W.; Li, S.K.; Wu, W.J. The main chemical composition and in vitro antifungal activity of the essential oils of Ocimum basilicum Linn. var. pilosum (Willd.) Benth. Molecules 2009, 14, 273–278. [Google Scholar]
- Sample Availability: Samples of the compounds S1–S8 and C1–C8 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, X.-J.; Miao, F.; Yao, Y.; Cao, F.-J.; Yang, R.; Ma, Y.-N.; Qin, B.-F.; Zhou, L. In Vitro Antifungal Activity of Sanguinarine and Chelerythrine Derivatives against Phytopathogenic Fungi. Molecules 2012, 17, 13026-13035. https://doi.org/10.3390/molecules171113026
Yang X-J, Miao F, Yao Y, Cao F-J, Yang R, Ma Y-N, Qin B-F, Zhou L. In Vitro Antifungal Activity of Sanguinarine and Chelerythrine Derivatives against Phytopathogenic Fungi. Molecules. 2012; 17(11):13026-13035. https://doi.org/10.3390/molecules171113026
Chicago/Turabian StyleYang, Xin-Juan, Fang Miao, Yao Yao, Fang-Jun Cao, Rui Yang, Yan-Ni Ma, Bao-Fu Qin, and Le Zhou. 2012. "In Vitro Antifungal Activity of Sanguinarine and Chelerythrine Derivatives against Phytopathogenic Fungi" Molecules 17, no. 11: 13026-13035. https://doi.org/10.3390/molecules171113026