2. Results and Discussion
Spiculisporic acid B (
1) was isolated as a white solid, with the molecular formula C
17H
26O
6 (five degrees of unsaturation) as derived from ESI high-resolution mass spectrometry ([M−H]
− at
m/z 325.1664, calculated 325.1657) and
1H- and
13C-NMR spectral data (
Table 1 and
Table 2). The
13C-NMR showed three carbonyl carbons at
δC 178.5, 175.5, and 174.2, one olefinic methine carbon at
δC 140.2, one olefinic methylene carbon at
δC 114.8, one oxygen bearing quaternary carbon at
δC 88.2, one methine carbon at
δC 52.5, and ten aliphatic carbons in the upfield (
δC 35.0 to 28.9) region. The
1H-NMR spectrum displayed signals of one terminal vinyl group at
δH 5.81 (ddt,
J = 16.1, 10.2, 6.8 Hz, H-14), 4.98 (br d,
J = 16.1 Hz, H-15a), and 4.91 (br d,
J = 10.2 Hz, H-15b), and 21 aliphatic protons. Together, these data indicate that compound
1 has one double bond and three carbonyls, which account for 4 out of the 5 degrees of unsaturation required by the molecular formula, so spiculisporic acid B must contain a ring. The structural information for
1 was determined from a series of 2D NMR analyses, including HSQC,
1H-
1H COSY, and HMBC spectra (
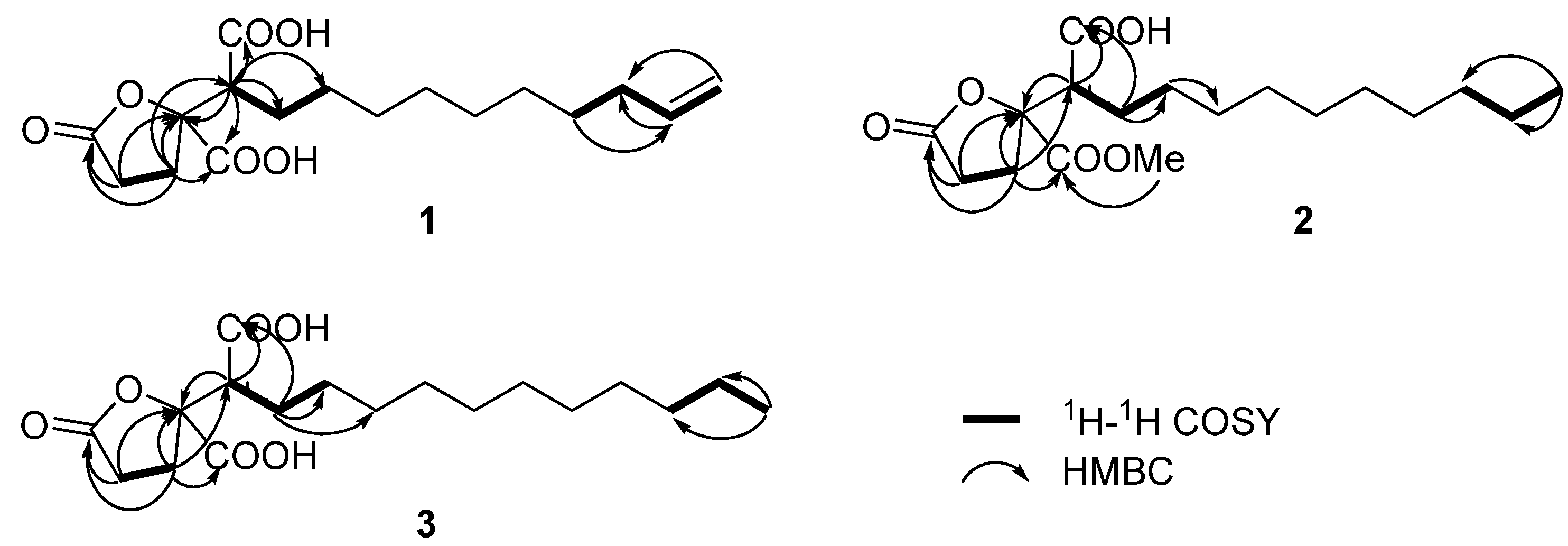
Figure 2). The
1H-
1H COSY experiment revealed a correlation between H-2 (
δH 2.60) and H-3 (
δH 2.49), and a seperate spin system, H
2C=CH-CH
2-(CH
2)
6-CH
2-CH-. The methine proton H-14 was coupled with the methylene protons H-13 (
δH 2.04) and H-15, and the methylene proton H-13 was coupled with the methylene proton at H-12 (
δH 1.37). The correlations between the methylene proton H-6a (
δH 1.85) and H-5 [
δH 3.01 (br d,
J = 9.2 Hz)] and H-7 (
δH 1.25–1.37) were observed in the
1H-
1H COSY spectrum. HMBC correlations from H-5 to C-4 (
δC 88.2) and two carbonyl carbons (
δC 175.5 and 174.2), from H-3 to C-1 (
δC 178.5), C-4, C-5 (
δC 52.5), and one of the carbonyl carbons (
δC 174.2), and from H-2 to C-1 and C-4, were observed. These observations allowed the structure of
1 to be determined as shown in
Figure 1.
Table 1.
1H-NMR spectral data (500 MHz, CD3OD) of compounds 1–3.
Table 1.
1H-NMR spectral data (500 MHz, CD3OD) of compounds 1–3.
| Position | 1 | 2 | 3 |
|---|
| 2 | 2.60 (m) | 2.59 (m) | 2.59 (m) |
| 3 | 2.49 (m) | 2.48 (m) | 2.46 (m) |
| 4-COOMe | | 3.80 (s) | |
| 5 | 3.01 (br d,
J = 9.2) | 2.97 (dd,
J = 10.8, 2.5) | 3.03 (dd,
J = 11.0, 3.0) |
| 6 | 1.85 (m); 1.50 (m) | 1.82 (m); 1.51 (m) | 1.85 (m); 1.53 (m) |
| 7 | 1.25–1.37 (m)
a | 1.43 (m); 1.32 (m) | 1.42 (m); 1.32 (m) |
| 8 | 1.25–1.37 (m)
a | 1.25–1.37 (m)
b | 1.25–1.38 (m)
c |
| 9 | 1.25–1.37 (m)
a | 1.25–1.37 (m)
b | 1.25–1.38 (m)
c |
| 10 | 1.25–1.37 (m)
a | 1.25–1.37 (m)
b | 1.25–1.38 (m)
c |
| 11 | 1.25–1.37 (m)
a | 1.25–1.37 (m)
b | 1.25–1.38 (m)
c |
| 12 | 1.37 (m) | 1.25–1.37 (m)
b | 1.25–1.38 (m)
c |
| 13 | 2.04 (m) | 1.25–1.37 (m)
b | 1.25–1.38 (m)
c |
| 14 | 5.81 (ddt,
J = 16.1, 10.2, 6.8) | 1.25–1.37 (m)
b | 1.25–1.38 (m)
c |
| 15 | 4.98 (br d, J = 16.1)
4.91 (br d, J = 10.2) | 0.90 (t,
J = 6.8) | 1.25–1.38 (m)
c |
| 16 | | | 0.90 (t,
J = 7.0) |
Table 2.
13C-NMR spectral data (125 MHz, CD3OD) of compounds 1–3.
Table 2.
13C-NMR spectral data (125 MHz, CD3OD) of compounds 1–3.
| Position | 1 | 2 | 3 |
|---|
| 1 | 178.5 (s) | 178.0 (s) | 178.7 (s) |
| 2 | 28.9 (t)
a | 28.7 (t) | 29.0 (t)
e |
| 3 | 30.3 (t) | 30.5 (t) | 30.6 (t)
d |
| 4 | 88.2 (s) | 88.2 (s) | 88.7 (s) |
| 4-COOH | 174.2 (s) | | 174.9 (s) |
| 4-CO | | 172.8 (s) | |
| OMe | | 53.6 (q) | |
| 5 | 52.5 (d) | 52.9 (d) | 52.7 (d) |
| 5-COOH | 175.5 (s) | 175.2 (s) | 175.8 (s) |
| 6 | 29.2 (t) | 28.9 (t)
b | 29.1 (t) |
| 7 | 28.9 (t)
a | 28.9 (t)
b | 29.0 (t)
e |
| 8 | 30.7 (t)
f | 30.5 (t)
c,g | 30.6 (t)
d |
| 9 | 30.4 (t)
f | 30.4 (t)
g | 30.6 (t)
d,h |
| 10 | 30.5 (t)
f | 30.5 (t)
c,g | 30.8 (t)
h |
| 11 | 30.5 (t)
f | 30.8 (t)
g | 30.9 (t)
h |
| 12 | 30.2 (t) | 30.7 (t)
g | 30.7 (t)
h |
| 13 | 35.0 (t) | 33.1 (t) | 30.5 (t) |
| 14 | 140.2 (d) | 23.8 (t) | 33.2 (t) |
| 15 | 114.8 (t) | 14.5 (q) | 23.8 (t) |
| 16 | | | 14.5 (q) |
Figure 2.
1H-1H COSY and selected HMBC correlations of compounds 1–3.
Figure 2.
1H-1H COSY and selected HMBC correlations of compounds 1–3.
To confirm the absolute configuration of
1, through comparison of its chemical shifts for C-4 (
δC 88.2) and C-5 (
δC 52.5) with those of the known compounds (−)-spiculisporic acid (
δC 88.1, 52.5) and (−)-epispiculisporic acid (
δC 88.0, 51.9) [
7,
8], we tentatively propose the absolute configuration at C-4 and C-5 in
1 to be 4
S and 5
S as (−)-spiculisporic acid. Thus, the structure was identified as (4
S,5
S)-4-(5-carboxyl-undecyl-14-enyl)-1-oxo-tetrahydrofuran-4-carboxyl acid, named spiculisporic acid B.
Spiculisporic acid C (
2) was obtained as a waxy solid that analyzed for the molecular formula C
18H
30O
6 by HR-ESI-MS data ([M+Na]
+ m/z 365.1927), and by comprehensive analysis of NMR data. This formula differed by the addition of CH
2 to the molecular formula of spiculisporic acid [
7,
8], suggesting an additional methylene or methyl group had been added to the structure. The
1H and
13C-NMR data for
2 were almost identical to those of spiculisporic acid, except for the presence of a new oxygenated methyl group (
δC 53.6,
δH 3.80). The HMBC spectrum showed a strong correlation between the oxygenated methyl protons and carbonyl carbon at
δC 172.8, which was correlated with H-3 (
δH 2.48), thus indicating the position of the methoxyl group. Based on the HSQC,
1H-
1H COSY, and HMBC analyses of
2 (
Figure 2), and the good comparison of NMR data of C-4 (
δC 88.2) and C-5 (
δC 52.9) in
2 to those from (−)-spiculisporic acid, we proposed the structure of
2 to be (4
S,5
S)-4-(5-carboxyl-undecyl)-1-oxo-tetrahydrofuran-4-carboxyl acid methyl ester, named spiculisporic acid C. It was possible that the methoxyl group in
2 was a result of a reaction with methanol in the procedure of isolation.
Spiculisporic acid D (
3), isolated as a pale white solid, gave a [M+Na]
+ ion peak at
m/z 365.1940 in its positive-mode HR-ESI-MS, indicating its molecular formula to be C
18H
30O
6, which was the same as that of
2. Through detailed analyses of the
1H- and
13C-NMR spectra of
3, the major difference between
3 and spiculisporic acid was the presence of a new methylene group in the aliphatic chain. Unambiguous assignments of
1H- and
13C-NMR data were obtained by interpretation of HSQC,
1H-
1H COSY, and HMBC data (
Figure 2), confirming the structure for
3 as shown. The absolute configuration of
3, determined by comparison of NMR data of C-4 (
δC 88.7) and C-5 (
δC 52.7) in
3with those of (−)-spiculisporic acid, was elucidated as (4
S,5
S)-4-(5-carboxyl-dodecyl)-1-oxo-tetrahydrofuran-4-carboxyl acid, named spiculisporic acid D.
Compounds
1–
3 were subjected to cytotoxic activity tests against two cell lines, SGC-7901 and SPC-A-1 by MTT methods [
9]. However, none of these compounds were active with IC
50 > 50 μg/mL. Compounds
1–
3 showed antibacterial activities against
Staphylococcus aureus ATCC 51650 with inhibition zone of 9.6, 11.6, and 11.5 mm at 20 mg/mL, while the diameter of inhibition zone of the positive control was 23.6 mm. Spiculisporic acid, a fermentation adduct from the culture broth of
Penicillium spiculisporum has found potential use as new controlled release carriers of active chemicals [
7], and commercial application as a biosurfactant for metal decontamination processes to remove hard, large metal cations from water [
10]. These interesting properties of spiculisporic acids B–D are currently under investigation.
3. Experimental
3.1. General Experimental Procedures
Optical rotations were taken on a Rudolph Autopol III. UV spectra were measured on a Hitachi U-3000 spectrophotometer, and IR spectra (KBr) were obtained on a Nicolet 380 FT-IR spectrometer. NMR spectra were recorded on a Bruker AVIII-500 spectrometer at 500 MHz for 1H-NMR and at 125 MHz for 13C-NMR. Chemical shifts are given in δ (ppm) and referenced to the solvent signal (methanol-d4, δH 3.31, δC 49.1) as the internal standard, and coupling constants (J) are reported in Hz. HR-ESI-MS spectra were recorded on a Agilent 6210 TOF LC/MS mass spectrometer. Silica gel (200–300 mesh) for column chromatography (CC) and silica GF254 (10–20 mm) for TLC were obtained from Qingdao Marine Chemical Factory (Qingdao, China). YMC ODS gel (50 μm) was purchased from Shanghai HANKING Instrument & Equipment Co., Ltd. (Shanghai, China) Sephadex LH-20 for chromatography was purchased from Merck (Darmstadt, Germany). Semipreparative HPLC was performed on a Hitachi L-7110 pump, and UV detector L-7400 equipped with a Waters ODS column (5 μm, 250 × 4.6 mm).
3.2. Fungal Material and Cultivation
The fungus Aspergillus sp. HDf2 was isolated and identified by one of the authors (R.W.) from the gut of a healthy sea urchin Anthocidaris crassispina collected from the seashore of Qionghai, Hainan, China, in October 2009. A voucher specimen with the code HNF-HD02 is deposited in the Hainan Provincial Fisheries Research Institute. The fungus was cultivated on MEA solid medium composed of 20 g/L malt extract, 20 g/L sucrose, 1 g/L peptone, 20 g/L agar and deionized water for 5 days at 28 °C. Agar plugs were used to inoculate in 1000-mL Erlenmeyer flasks, each containing 300 mL of ME liquid media. Fermentation was carried out on a rotary shaker (140 rpm) at 26 °C for 12 days in 40 × 1,000 mL Erlenmeyer flasks.
3.3. Extraction and Isolation
The filtrate (12 L) of the fermented culture broth was extracted three times with EtOAc (12 L × 4) at room temperature, and the organic solvent was evaporated to dryness under reduced pressure to afford a yellow crude extract (4.1 g), which was subjected to silica gel (41 g, 200–300 mesh) CC (4 × 75 cm) eluted with a gradient of CHCl3–MeOH (v/v 100:0, 100:1, 100:2, 100:4, 100:8, 100:16 and 0:100, each 600 mL) to give seven fractions. The CHCl3–MeOH (100:4) fraction (710.3 mg) was further purified by Sephadex LH-20 CC (1.5 × 30 cm) eluting with MeOH (500 mL) and then by ODS CC (2.5 × 40 cm) with a gradient of MeOH-H2O (v/v 50:50, 65:35, 80:20, 100:0, each 400 mL) to afford a fraction (110.5 mg) (MeOH–H2O, 80:20) containing 1–3, which were purified by semipreparative reversed-phase HPLC [2 mL/min; MeOH-0.1% TFA in H2O (78:22)] (1, 10.3 mg, tR = 16.0 min; 2, 16.2 mg, tR = 27.8 min; 3, 22.6 mg, tR = 29.6 min). All these compounds were stored at 4 °C.
Spiculisporic acid B (
1): (4
S,5
S)-4-(5-Carboxylundecyl-14-enyl)-1-oxo-tetrahydrofuran-4-carboxylic acid. White solid; [
α]
30D = −4.8 (c = 0.028, EtOH); UV (MeOH)
λmax (log
ε): 199 (3.07), 215 (3.89) nm; IR (KBr)
νmax: 2912, 2853, 1711, 1688, 1415, 1272, 1175, 932 cm
−1;
1H and
13C-NMR spectral data are listed in
Table 1 and
Table 2; HR-ESI-MS:
m/z 325.1664 [M−H]
− (calculated for C
17H
25O
6, 325.1657).
Spiculisporic acid C (
2): (4
S,5
S)-4-(5-Carboxylundecyl)-1-oxo-tetrahydrofuran-4-carboxylic acid methyl ester. Waxy solid; [
α]
30D = −24.7 (c = 0.078, EtOH); UV (MeOH)
λmax (log
ε): 214 (3.18) nm; IR (KBr)
νmax: 2921, 2855, 1716, 1663, 1412, 1274, 1183, 952 cm
−1;
1H and
13C-NMR spectral data are listed in
Table 1 and
Table 2; HR-ESI-MS:
m/z 365.1927 [M+Na]
+ (calculated for C
18H
30O
6Na, 365.1935).
Spiculisporic acid D (
3): (4
S,5
S)-4-(5-Carboxyldodecyl)-1-oxo-tetrahydrofuran-4-carboxylic acid. Pale white solid; [
α]
30D = −11.8 (c = 0.028, EtOH); UV (MeOH)
λmax (log
ε): 212 (3.22) nm; IR (KBr)
νmax: 2918, 2860, 1721, 1657, 1423, 1268, 1175, 944 cm
−1;
1H and
13C-NMR spectral data are listed in
Table 1 and
Table 2; HR-ESI-MS:
m/z 365.1940 [M+Na]
+ (calculated for C
18H
30O
6Na, 365.1935).
3.4. In Vitro Cytotoxicity Test
The cytotoxic activities for compounds
1–
3 were tested
in vitro against two cell lines, SGC-7901 (human gastric adenocarcinoma) and SPC-A-1 (human lung adenocarcinoma), which were purchased from the Jiangsu Provincial Center for Disease Prevention and Control. The purity of the tested compounds and doxorubicin·HCl was determined to be over 95% by using the HPLC-DAD method. The cytotoxic
in vitro effects on these tested cell were assessed by the IC
50 values, and determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colometric method [
8]. Each set of test was conducted three times to confirm reproducibility of the results. The compounds were dissolved in DMSO (dimethyl sulfoxide). Doxorubicin·HCl was used as a positive control, and the medium without test compound as a negative control in the bioassay.
3.5. Antibacterial Test
Compounds 1–3 were tested for in vitro antimicrobial activity against Staphylococcus aureus ATCC51650 by the filter paper disc agar diffusion method. The NA medium was mixed with 2 mL of suspension containing 1 × 105 ~ 1 × 107 cfu/mL of Staphylococcus aureus, and then poured into petri-plates. 2 μL 20 mg/mL of the isolated compounds dissolved in DMSO were impregnated on sterile filter paper discs (6 mm diameter) and then were applied on the surface of the solidified agar plates. Every sample was tested in triplicate. Streptomycin sulfate (2 μL, 20 mg/mL) was used as positive control. The test plates were incubated at 37 °C for 24 h. Then the diameters of the inhibition zones including the 6 mm disc diameter were measured.