New Furanones from the Plant Endophytic Fungus Pestalotiopsis besseyi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation
| Position | 1 a | 2 a | 3 a | 4 a | 5 a |
|---|---|---|---|---|---|
| 5 | 4.65, s | 4.85, s | 4.63, s | 4.78, s | 4.78, d (17) |
| 4.71, d (17) | |||||
| 6 | 6.10, d (16) | 6.26, d (16) | 2.45, t (7.5) | 6.24, d (16) | 2.61, t (7.5) |
| 7 | 6.86, m | 6.97, m | 1.52, m | 6.85, m | 1.55, m |
| 8 | 1.86, d (7.0) | 1.89, d (6.7) | 0.93, t (7.5) | 1.81, d (6.5) | 0.94, t (7.5) |
| 9 | 2.70, m | 6.77, d (16) | 2.67, br s | 2.90, dd (15, 3.6) | 2.86, dd (12, 4.0) |
| 2.65, dd (15, 6.0) | 2.44, dd (12, 7.0) | ||||
| 10 | 2.70, m | 6.04, dd (16, 7.7) | 2.67, br s | 2.77, m | 2.75, ddd (7.0, 4.0, 2.0) |
| 11 | 4.52, m | 2.85, dq (4.5, 2.0) | 2.81, dq (7.5, 2.0) | ||
| 11-OH | 1.75, br s | ||||
| 12 | 2.18, s | 1.68, d (7.0) | 2.19, s | 1.24, d (4.5) | 0.93, d (7.5) |
| Position | 1 a | 2 a | 3 a | 4 a | 5 a | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 172.9, qC | 173.0, qC | 177.5, qC | 173.0, qC | 174.5, qC | ||||
| 3 | 123.3, qC | 122.7, qC | 127.9, qC | 123.9, qC | 128.7, qC | ||||
| 4 | 156.6, qC | 149.4, qC | 158.9, qC | 156.3, qC | 158.5, qC | ||||
| 5 | 70.8, CH2 | 68.6, CH2 | 71.2, CH2 | 71.7, CH2 | 71.8, CH2 | ||||
| 6 | 118.1, CH | 118.7, CH | 25.6 CH2 | 119.9, CH | 25.6, CH2 | ||||
| 7 | 133.0, CH | 134.3, CH | 20.6 CH2 | 132.3, CH | 21.4, CH2 | ||||
| 8 | 19.3, CH3 | 19.6, CH3 | 13.9, CH3 | 17.4, CH3 | 13.9, CH3 | ||||
| 9 | 20.4, CH2 | 118.6, CH | 21.3, CH2 | 30.2, CH2 | 29.9 CH2 | ||||
| 10 | 41.0, CH2 | 140.8, CH | 41.3, CH2 | 57.2, CH | 56.8, CH | ||||
| 11 | 206.1, qC | 68.3, CH | 206.0, qC | 54.7, CH | 54.5, CH | ||||
| 12 | 29.8, CH3 | 23.5, CH3 | 29.8, CH3 | 19.1, CH3 | 17.2, CH3 |
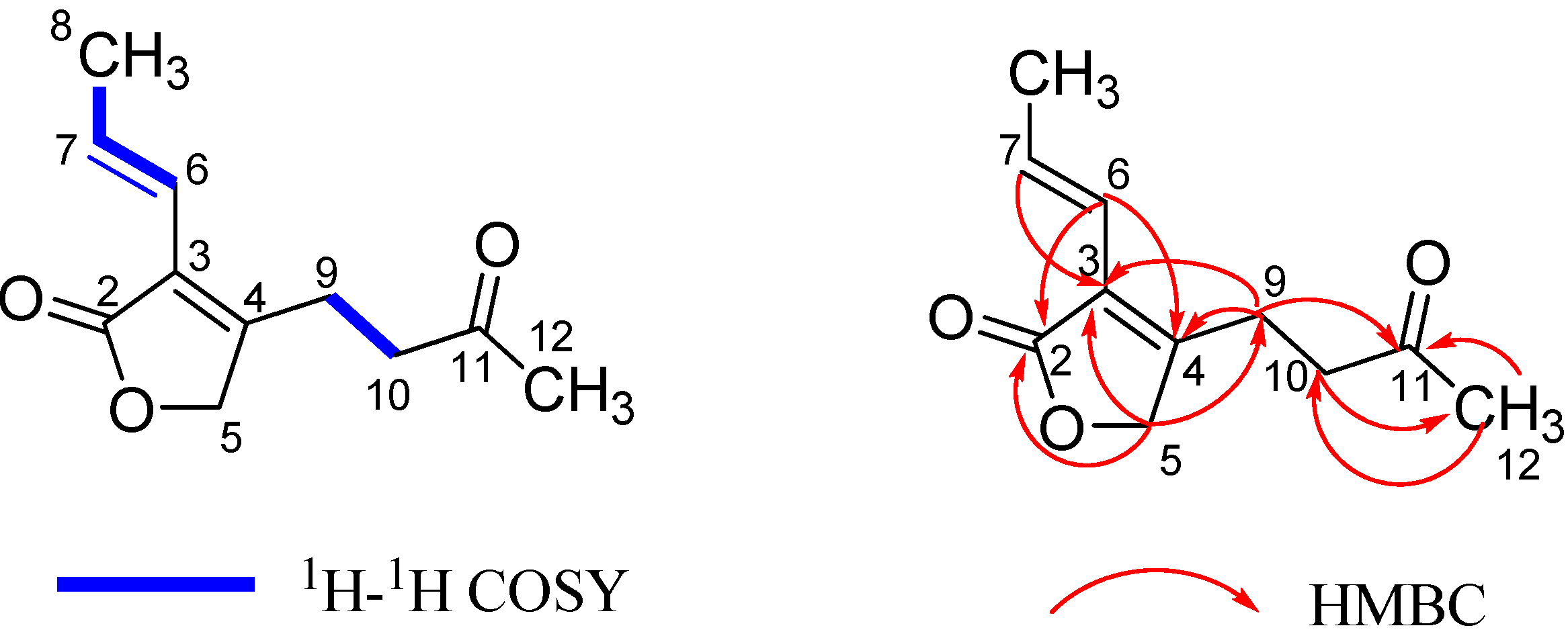
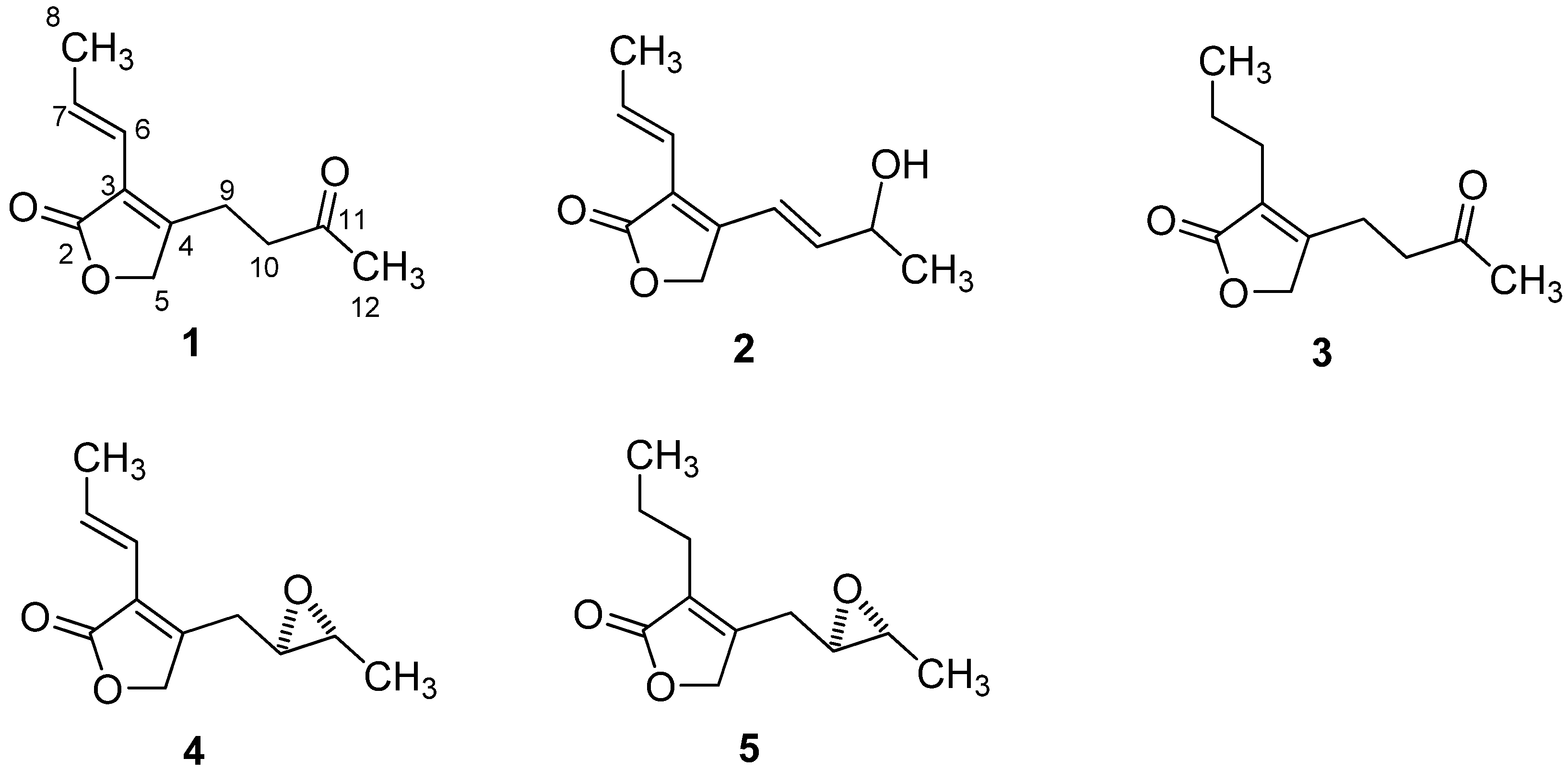
2.2. Anti-HIV Activity
2.3. Antifungal Activity
3. Experimental
3.1. General
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. Anti-HIV Assay
3.5. Antifungal Bioassay
4. Conclusions
Acknowledgments
References
- Suryanarayanan, T.S.; Thirunavukkarasu, N.; Govindarajulu, M.B.; Sasse, F.; Jansen, R.; Murali, T.S. Fungal endophytes and bioprospecting. Fungal Biol. Rev. 2009, 23, 9–19. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, J.Z.; Luo, D.Q. The taxonomy, Biology and chemistry of the fungal Pestalotiopsis genus. Nat. Prod. Rep. 2012, 29, 622–641. [Google Scholar] [CrossRef]
- Wang, K.W.; Lei, J.X.; Wei, J.G.; Yao, N. Bioactive natural compounds from the plant endophytic fungi Pestalotiopsis genus. Mini Rev. Med. Chem. 2012, 12, 1382–1393. [Google Scholar] [CrossRef]
- Xu, J.; Ebada, S.S.; Proksch, P. Pestalotiopsis a highly creative genus: Chemistry and bioactivity of secondary metabolites. Fungal Divers. 2010, 44, 15–31. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.D.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Pestalotiopsis—Morphology, Phylogeny, Biochemistry and diversity. Fungal Divers. 2011, 50, 167–187. [Google Scholar] [CrossRef]
- Tori, M.; Arbiyanti, H.; Taira, Z.; Asakawa, Y. Terpenoids of the liverwort Frullanoides densifolia and Trocholejeunea sandvicensis. Phytochemistry 1993, 32, 335–348. [Google Scholar] [CrossRef]
- Zhang, G.H.; Wang, Q.; Chen, J.J.; Zhang, X.M.; Tam, S.C.; Zheng, Y.T. The anti-HIV-1 effect of scutellarin. Biochem. Biophys. Res. Commun. 2005, 334, 812–816. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Fothergill, A.; Ghannoum, M.A.; Manavathu, E.; Ostrosky-Zeichner, L.; Pfaller, M.; Rinaldi, M.; Schell, W.; Walsh, T. Quality control and reference guidelines for CLSI broth micro-dilution method (M38-A document) for susceptibility testing of anidulafungin against moulds. J. Clin. Microbiol. 2007, 45, 2180–2182. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors because those compounds were used by bioactivity tests.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, H.; Liu, S.; Guo, L.; Zhang, Y.; Cui, L.; Ding, G. New Furanones from the Plant Endophytic Fungus Pestalotiopsis besseyi. Molecules 2012, 17, 14015-14021. https://doi.org/10.3390/molecules171214015
Liu H, Liu S, Guo L, Zhang Y, Cui L, Ding G. New Furanones from the Plant Endophytic Fungus Pestalotiopsis besseyi. Molecules. 2012; 17(12):14015-14021. https://doi.org/10.3390/molecules171214015
Chicago/Turabian StyleLiu, Haitao, Shuchun Liu, Liangdong Guo, Yonggang Zhang, Langjun Cui, and Gang Ding. 2012. "New Furanones from the Plant Endophytic Fungus Pestalotiopsis besseyi" Molecules 17, no. 12: 14015-14021. https://doi.org/10.3390/molecules171214015




