Antioxidant Phenolic Compounds from Pu-erh Tea
Abstract
:1. Introduction
2. Results and Discussion
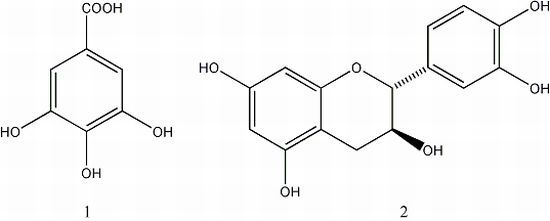
2.1. Elucidation of the Purified Compounds
2.2. Antioxidant Activity
3. Experimental
3.1. General Procedures and Reagents
3.2. Plant Materials
3.3. Extraction and Isolation of Antioxidant Compounds
3.4. Spectroscopic Data
3.5. DPPH˙ Scavenging Activity
3.6. ABTS˙+ Scavenging Activity
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Jeng, K.C.; Chen, C.S.; Fang, Y.P.; Hou, R.C.; Chen, Y.S. Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-erh tea. J. Agric. Food Chem. 2007, 55, 8787–8792. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, K.L.; Zhong, Y.; Luo, X.; Xiao, R.; Hou, Y. Acute and subchronic oral toxicities of Pu-erh black tea extract in Sprague-Dawley rats. J. Ethnopharmacol. 2011, 134, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Yang, C.R. Chemical constituents of crude green tea, the material of Pu-erh tea in Yunnan. Acta Bot. Yunnanica 2000, 22, 343–350. [Google Scholar]
- Zhou, Z.-H.; Zhang, Y.-J.; Xu, M.; Yang, C.-R. Puerins A and B, two new 8-C substituted flavan-3-ols from Pu-erh tea. J. Agric. Food Chem. 2005, 53, 8614–8617. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Takeuaka, Y.; Kojima, R.; Saito, S.I.; Tomita, I.; Katou, M.; Shibuya, S. Effects of Pu-erh tea on lipid metabolism in rats. Chem. Pharm. Bull. 1986, 34, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.D.; Yen, G.C.; Yen, W.J.; Wang, B.S.; Chang, L.W. Effects of Pu-erh tea on oxidative damage and nitric oxide scavenging. J. Agric. Food Chem. 2004, 52, 8169–8176. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.S.; Peng, C.X.; He, X.; Li, J.H.; Li, B.C.; Zhou, H.J. Antioxidant activity of extracts of Pu-erh tea and its material. Asian J. Agric. Sci. 2009, 1, 48–54. [Google Scholar]
- Qian, Z.M.; Guan, J.; Yang, F.Q.; Li, S.P. Identification and quantification of free radical scavengers in Pu-erh tea by HPLC-DAD-MS coupled online with 2,2'-azinobis(3-ethylbenzthiazolinesulfonic acid) diammonium salt assay. J. Agric. Food Chem. 2011, 82, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, B.L.; Hou, J.W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta 1999, 1427, 13–23. [Google Scholar] [CrossRef]
- Yokozawa, T.; Cho, E.J.; Hara, Y.; Kitani, K. Antioxidative activity of green tea treated with radical initiator 2,2'-azobis(2-amidinopropane) dihydrochloride. J. Agric. Food Chem. 2000, 48, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, F.; Ono, M.; Masuoka, C.; Ito, Y.; Sakata, Y.; Shimizu, K.; Nonaka, G.; Nishioka, I.; Nohara, T. Evaluation of the anti-oxidative effect (in vitro) of tea polyphenols. Biosci. Biotechnol. Biochem. 2003, 67, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [PubMed]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.R.; Sastry, V.G.; Bano, N. Antibacterial and antioxidant activities of some novel chalcone derivatives. J. Pharm. Res. 2011, 4, 2347–2349. [Google Scholar]
- Zou, Y.L.; Dong, B.S.; Zhang, F.Q.; He, M.; Li, C.; Ou, L.C.; He, Y.P. Chemical Constituents of Pu-erh tea. Yunnan Chem. Technol. 2009, 36, 10–13. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the crude extracts and pure compounds 1–8 are available from the authors. |


| Investigated materials | DPPH radical scavenging rate (%) | IC50 (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 200 (μg/mL) | 100 (μg/mL) | 50 (μg/mL) | 25 (μg/mL) | 12.50 (μg/mL) | 6.25 (μg/mL) | ||
| Water extract | 66.75 ± 1.96 b | 63.87 ± 1.92 b | 58.30 ± 2.00 b | 54.17 ± 1.57 d | 46.53 ± 1.26 c | 29.15 ± 0.94 d | 25.79 |
| 1 | 82.92 ± 2.58 a | 82.11 ± 3.01 a | 82.39 ± 3.58 a | 80.29 ± 3.41 a,b,c | 60.29 ± 1.70 a | 50.90 ± 1.70 b | 4.98 |
| 2 | 68.10 ± 2.30 b | 67.21 ± 2.08 b | 60.55 ± 2.61 b | 57.56 ± 2.41 d | 51.38 ± 1.55 b | 50.55 ± 1.44 b | 7.22 |
| 3 | 69.57 ± 2.25 b | 53.09 ± 1.60 c | 29.74 ± 1.11 c | 23.32 ± 1.08 e | 12.83 ± 0.46 d | 10.94 ± 0.28 e | 92.43 |
| 4 | 82.61 ± 3.14 a | 82.72 ± 2.96 a | 78.01 ± 2.60 a | 73.44 ± 2.30 c | 59.78 ± 2.08 a | 45.82 ± 1.12 c | 6.90 |
| 5 | 83.36 ± 2.40 a | 83.57 ± 2.87 a | 79.41 ± 3.95 a | 76.50 ± 2.59 b,c | 58.36 ± 2.14 a | 45.98 ± 1.40 c | 6.84 |
| 6 | 84.07 ± 1.79 a | 84.07 ± 3.28 a | 79.34 ± 3.65 a | 75.81 ± 3.07 b,c | 59.48 ± 2.30 a | 46.96 ± 1.07 c | 6.61 |
| 7 | 84.08 ± 3.00 a | 84.03 ± 2.61 a | 83.37 ± 3.38 a | 83.49 ± 3.16 a,b | 60.48 ± 1.79 a | 52.90 ± 1.44 b | 4.64 |
| 8 | 83.92 ± 1.87 a | 83.44 ± 2.53 a | 83.46 ± 3.34 a | 83.18 ± 3.02 a,b | 63.31 ± 2.79 a | 59.14 ± 1.13 a | 2.79 |
| Vitamin C | 84.72 ± 2.55 a | 83.83 ± 2.66 a | 85.20 ± 3.16 a | 85.40 ± 3.46 a | 51.10 ± 2.01 b | 45.61 ± 1.25 c | 7.50 |
| Investigated materials | ABTS radical scavenging rate (%) | IC50 (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 200 (μg/mL) | 100 (μg/mL) | 50 (μg/mL) | 25 (μg/mL) | 12.5 (μg/mL) | 6.25 (μg/mL) | ||
| Water extract | 71.34 ± 2.29 b | 58.72 ± 1.50 b | 54.94 ± 2.06 d | 51.23 ± 2.08 d | 42.07 ± 1.79 d | 27.54 ± 0.99 e | 32.30 |
| 1 | 95.45 ± 3.46 a | 95.59 ± 3.51 a | 94.48 ± 3.29 a | 94.31 ± 3.38 a | 83.43 ± 3.34 a | 60.12 ± 1.76 a | 2.97 |
| 2 | 94.24 ± 1.93 a | 94.40 ± 3.09 a | 93.38 ± 3.38 a,b | 93.33 ± 3.22 a | 78.15 ±2 .12 a | 60.11 ± 2.07 a | 3.12 |
| 3 | 69.75 ± 2.13 b | 51.77 ± 2.00 b | 27.74 ± 1.11 e | 24.58 ± 0.77 e | 15.45 ± 0.51 e | 10.08 ± 0.31f | 95.14 |
| 4 | 95.57 ± 2.89 a | 94.32 ± 3.03 a | 86.25 ± 2.55 b,c | 70.12 ± 1.93 c | 66.22 ± 2.00 b | 46.98 ± 1.58 d | 7.13 |
| 5 | 95.05 ± 3.91 a | 93.22 ± 2.77 a | 78.53 ± 2.39 c | 71.27 ± 2.18 c | 67.24 ± 2.03 b | 47.51 ± 1.29 d | 6.72 |
| 6 | 95.16 ± 4.04 a | 95.35 ± 3.34 a | 90.65 ± 2.83 a,b | 85.20 ± 2.95 b | 68.24 ± 1.93 b | 47.33 ± 1.30 d | 6.31 |
| 7 | 95.14 ± 4.18 a | 95.01 ± 3.85 a | 95.53 ± 3.29 a | 90.54 ± 2.79 a,b | 83.23 ± 2.40 a | 52.64 ± 1.38 b,c | 4.09 |
| 8 | 95.21 ± 2.81a | 94.38 ± 3.24 a | 93.66 ± 2.74 a,b | 90.77 ± 2.98 a,b | 80.61 ± 2.88 a | 53.33 ± 1.28 b | 4.04 |
| Vitamin C | 95.32 ± 3.15 a | 95.48 ± 3.45 a | 95.31 ± 2.92 a | 95.03 ± 2.97 a | 53.39 ± 1.61 c | 49.20 ± 1.37 c,d | 7.20 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, H.M.; Wang, C.F.; Shen, S.M.; Wang, G.L.; Liu, P.; Liu, Z.M.; Wang, Y.Y.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Antioxidant Phenolic Compounds from Pu-erh Tea. Molecules 2012, 17, 14037-14045. https://doi.org/10.3390/molecules171214037
Zhang HM, Wang CF, Shen SM, Wang GL, Liu P, Liu ZM, Wang YY, Du SS, Liu ZL, Deng ZW. Antioxidant Phenolic Compounds from Pu-erh Tea. Molecules. 2012; 17(12):14037-14045. https://doi.org/10.3390/molecules171214037
Chicago/Turabian StyleZhang, Hai Ming, Cheng Fang Wang, Sheng Min Shen, Gang Li Wang, Peng Liu, Zi Mu Liu, Yong Yan Wang, Shu Shan Du, Zhi Long Liu, and Zhi Wei Deng. 2012. "Antioxidant Phenolic Compounds from Pu-erh Tea" Molecules 17, no. 12: 14037-14045. https://doi.org/10.3390/molecules171214037