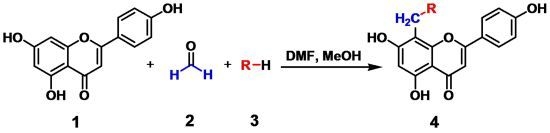
Nitrogen-Containing Apigenin Analogs: Preparation and Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
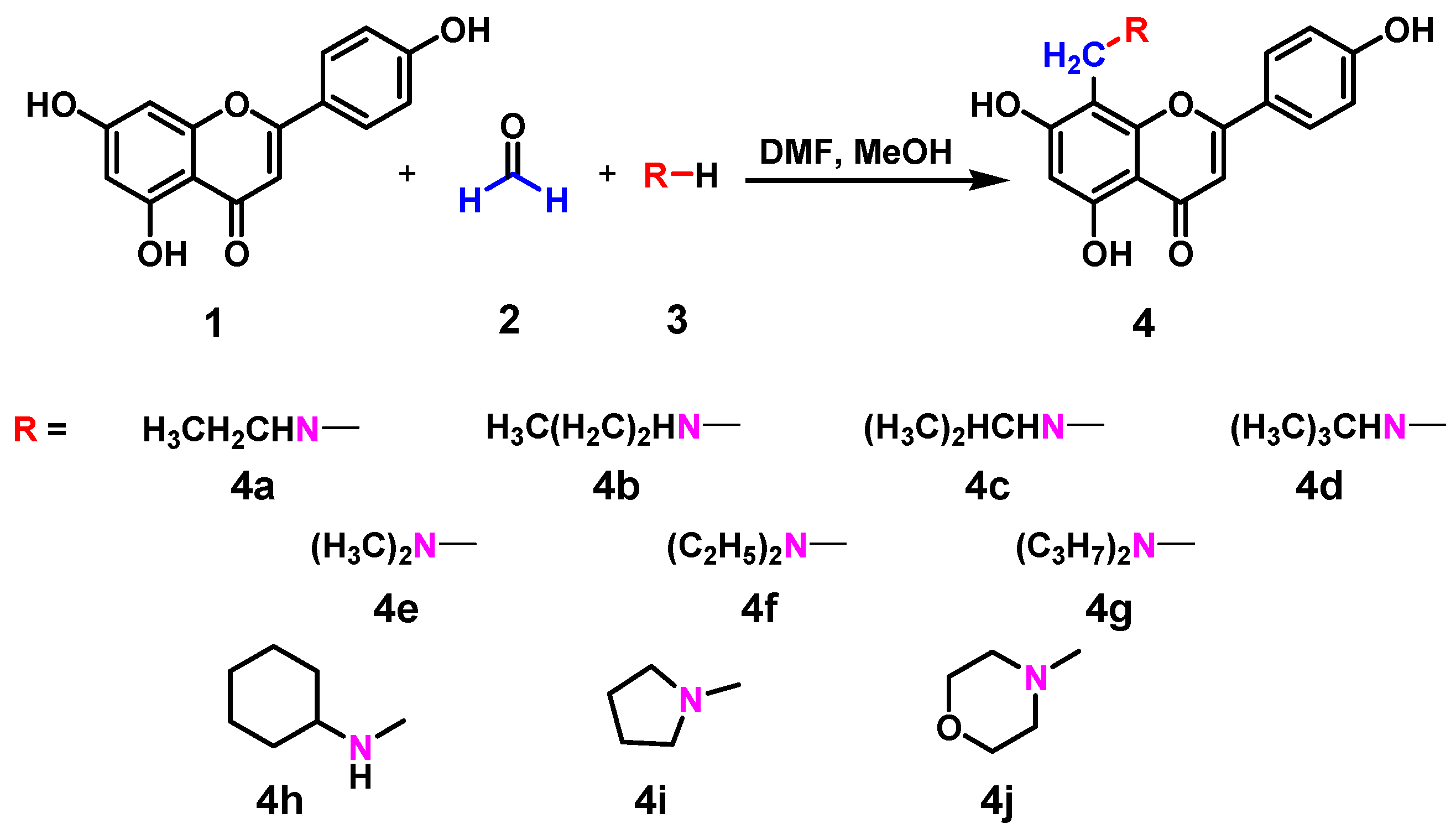
| Compounds | R | Time (h) | Temperature (°C) | Isolated yield (%) |
|---|---|---|---|---|
| 4a | Ethylamino | 4 | 25 | 40.5 |
| 4b | Propylamino | 12 | 30 | 38.7 |
| 4c | Isopropylamino | 4 | 35 | 61.7 |
| 4d | Tert-butylamino | 5 | 35 | 52.3 |
| 4e | Dimethylamino | 4 | 25 | 39.6 |
| 4f | Diethylamino | 1.5 | 25 | 60.9 |
| 4g | Diisopropylamino | 12 | 25 | 51.2 |
| 4h | Cyclohexylamino | 8 | 40 | 41.8 |
| 4i | Pyrrolidinyl | 1.5 | 40 | 42.1 |
| 4j | Morpholinyl | 12 | 40 | 43.4 |
2.2. Antibacterial Activity
| Compounds | S. aureus | B. subtilis | E. coli | P. aeruginosa |
|---|---|---|---|---|
| ATCC 25923 | ATCC 6633 | ATCC 25922 | ATCC 27853 | |
| Apigenin | 11.1 ± 0.3 | 11.0 ± 1.0 | 10.1 ± 0.3 | 10.3 ± 0.2 |
| 4a | 17.5 ± 0.1 | 21.3 ± 0.6 | 10.3 ± 0.1 | 8.6 ± 0.1 |
| 4b | 17.9 ± 0.6 | 17.5 ± 0.4 | 10.9 ± 0.7 | 10.6 ± 0.2 |
| 4c | 14.1 ± 0.5 | 15.2 ± 0.1 | 12.5 ± 0.1 | 10.5 ± 0.4 |
| 4d | 14.8 ± 0.2 | 18.0 ± 0.3 | 12.8 ± 0.4 | 10.8 ± 0.1 |
| 4e | 12.1 ± 0.6 | 15.9 ± 0.6 | 10.4 ± 0.2 | 12.8 ± 0.5 |
| 4f | 18.3 ± 0.1 | 18.7 ± 0.5 | 14.8 ± 0.5 | 12.4 ± 0.3 |
| 4g | 15.0 ± 0.3 | 19.0 ± 0.1 | 11.0 ± 0.3 | 10.0 ± 0.7 |
| 4h | 22.1 ± 0.5 | 22.8 ± 0.2 | 15.3 ± 0.1 | 17.0 ± 1.0 |
| 4i | 18.8 ± 0.4 | 19.3 ± 0.4 | 13.0 ± 0.1 | 10.2 ± 0.8 |
| 4j | 25.9 ± 0.6 | 27.2 ± 0.8 | 15.6 ± 0.3 | 14.3 ± 1.0 |
| Ampicillin | 37.3 ± 0.6 | 36.7 ± 1.5 | 28.9 ± 0.1 | 33.7 ± 0.5 |
| Tetracycline | 26.3 ± 0.5 | 21.0 ± 1.0 | 21.7 ± 1.1 | 24.3 ± 1.1 |
| Compounds | IC50 (μg/mL) a | MIC (μg/mL) a | |||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. aeruginosa | ||
| ATCC 25923 | ATCC 6633 | ATCC 25922 | ATCC 27853 | ||
| Apigenin | 1094.7 ± 2.2 | 31.25 | 31.25 | 62.5 | 62.5 |
| 4a | 788.0 ± 1.6 | 7.81 | 3.91 | 62.5 | 125 |
| 4b | 782.4 ± 2.3 | 7.81 | 7.81 | 62.5 | 62.5 |
| 4c | 670.1 ± 1.5 | 15.63 | 15.63 | 31.25 | 62.5 |
| 4d | 539.5 ± 1.4 | 15.63 | 7.81 | 31.25 | 62.5 |
| 4e | 542.3 ± 1.3 | 31.25 | 15.63 | 62.5 | 31.25 |
| 4f | 434.8 ± 1.4 | 7.81 | 7.81 | 15.63 | 31.25 |
| 4g | 431.1 ± 1.1 | 15.63 | 7.81 | 62.5 | 62.5 |
| 4h | 887.7 ± 2.5 | 3.91 | 3.91 | 15.63 | 15.63 |
| 4i | 334.8 ± 1.2 | 7.81 | 7.81 | 31.25 | 62.5 |
| 4j | 740.1 ± 1.8 | 1.95 | 1.95 | 15.63 | 15.63 |
| Ampicillin | -- | 0.06 | 0.12 | 0.98 | 0.49 |
| Tetracycline | -- | 1.95 | 3.91 | 3.91 | 3.91 |
| Vitamin C | 44.2 ± 1.1 | ||||
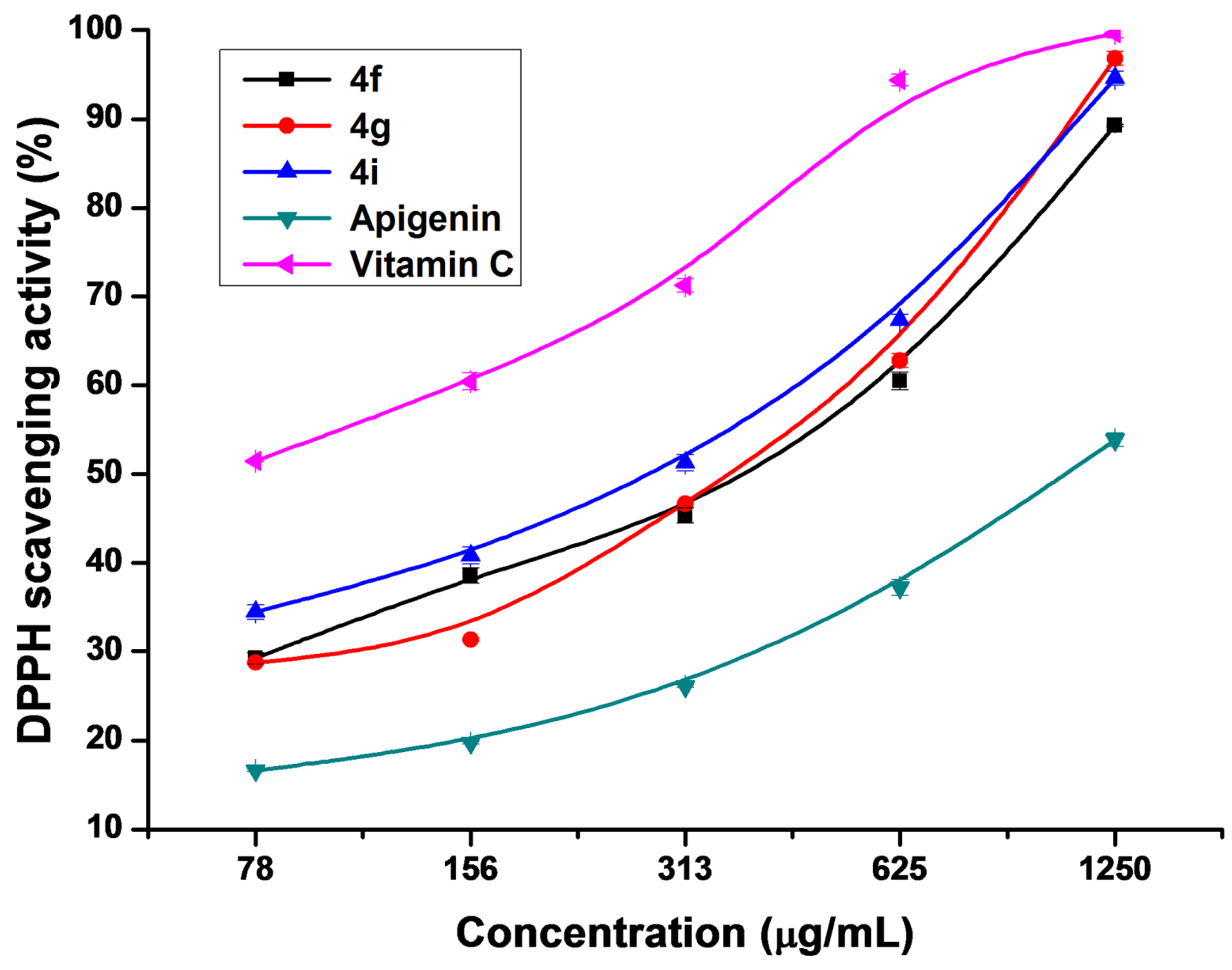
2.3. Antioxidant Activity
2.4. Antiproliferative Activity

| Compounds | IC50 (μg/mL) | |||
|---|---|---|---|---|
| HeLa | HepG2 | A549 | MCF-7 | |
| 1 | 450 ± 2.0 | 460 ± 2.2 | 1740 ± 3.4 | >2000 ± 3.6 |
| 4a | 450 ± 2.5 | 470 ± 2,7 | >2000 ± 4.3 | >2000 ± 4.1 |
| 4b | 430 ± 1.9 | 410 ± 2.2 | 1410 ± 3.0 | 420 ± 2.3 |
| 4c | 270 ± 1.4 | 270 ± 1.9 | >2000 ± 3.8 | 280 ± 1.7 |
| 4d | 570 ± 2.0 | 620 ± 3.1 | >2000 ± 4.0 | 310 ± 2.1 |
| 4e | 230 ± 1.6 | 270 ± 2.0 | 380 ± 2.8 | 210 ± 1.9 |
| 4f | 160 ± 1.5 | 150 ± 1.7 | >2000 ± 3.7 | 250 ± 2.0 |
| 4g | 210 ± 2.1 | 190 ± 1.5 | >2000 ± 4.2 | 610 ± 3.2 |
| 4h | 170 ± 1.7 | 180 ± 1.4 | >2000 ± 3.5 | >2000 ± 4.0 |
| 4i | 40 ± 1.8 | 40 ± 1.5 | 223 ± 2.1 | 166 ± 1.8 |
| 4j | 180 ± 2.0 | 220 ± 2.0 | >2000 ± 4.4 | 1010 ± 2.9 |
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Compounds 4a to 4j
3.3. Antibacterial Assay
3.4. Antioxidant Assay
3.5. Antiproliferative Assay
4. Conclusions
Acknowledgments
References
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar]
- Ren, W.Y.; Qiao, Z.H.; Wang, H.W.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Carlo, G.D.; Mascolo, N.; lzzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Ohemeng, K.A.; Schwender, C.F.; Fu, K.P.; Barrett, J.F. DNA gyrase inhibitory and antibacterial activity of some flavones (1). Bioorg. Med. Chem. Lett. 1993, 3, 225–230. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Bernard, F.X.; Sable, S.; Cameron, B.; Provost, J.; Desnottes, J.F.; Crouzet, J.; Blanche, F. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob. Agents Chemother. 1997, 41, 992–998. [Google Scholar]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. BBA Biomembranes 1993, 1147, 132–136. [Google Scholar]
- Tsuchiya, H.; Iinuma, M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar]
- Shahidi, F.; Ho, C.T. Phenolics in food and natural health products: An Overview. In Phenolic Compounds in Foods and Natural Health Products; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2005; pp. 1–8. [Google Scholar]
- Bandele, O.J.; Osheroff, N. Bioflavonoids as poisons of human topoisomerase IIα and IIβ. Biochemistry 2007, 46, 6097–6108. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Kawai, M.; Hirano, T.; Higa, S.; Arimitsu, J.; Maruta, M.; Kuwahara, Y.; Ohkawara, T.; Hagihara, K.; Yamadori, T.; Shima, Y.; et al. Flavonoids and related compounds as anti-allergic substances. Allergol. Int. 2007, 56, 113–123. [Google Scholar] [CrossRef]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.H.; Kang, E.S.; Kim, S.K. Antidiabetic agents from medicinal plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Sanchez, I.; Gómez-Garibay, F.; Taboada, J.; Ruiz, B.H. Antiviral effect of flavonoids on the dengue virus. Phytother. Res. 2000, 14, 89–92. [Google Scholar] [CrossRef]
- Park, K.Y.; Jung, G.O.; Lee, K.T.; Choi, M.Y.; Kim, G.T.; Jung, H.J.; Park, H.J. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J. Ethnopharmacol. 2004, 90, 73–79. [Google Scholar] [CrossRef]
- Anton, R.; Beretz, A. Flavonoids: Antithrombotic agents or nutrients. Bull. Acad. Natl. Med. 1990, 174, 709–714. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Kayan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Oh, H.; Kim, D.H.; Cho, J.H.; Kim, Y.C. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J. Ethnopharmacol. 2004, 95, 421–424. [Google Scholar] [CrossRef]
- Alscher, R.G.; Hess, J.L. Antioxidants in Higher Plants; CRC Press: Boca Raton, FL, USA, 1993; pp. 135–139. [Google Scholar]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostag. Leukotr. Ess. 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Chaumontet, C.; Droumaguet, C.; Bex, V.; Heberden, C.; Gaillard-Sanchez, I.; Martel, P. Flavonoids (apigenin, tangeretin) counteract tumor promoter-induced inhibition of intercellular communication of rat liver epithelial cells. Cancer Lett. 1997, 114, 207–210. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hisama, M. Antimutagenic activity of flavonoids from Chrysanthemum morifolium. Biosci. Biotechnol. Biochem. 2003, 67, 2091–2099. [Google Scholar] [CrossRef]
- Kuo, M.L.; Yang, N.C. Reversion of vH-ras-trasformed NIH 3T3 cells by apigenin through inhibiting mitogen-activated protein kinase and its downstream oncogenes. Biochem. Biophys. Res. Commun. 1995, 212, 767–775. [Google Scholar] [CrossRef]
- Fotsis, T.; Pepper, M.S.; Aktas, E.; Breit, S.; Rasku, S.; Adlercreutz, H.; Wähälä, K.; Montesano, R.; Schweigerer, L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997, 57, 2916–2921. [Google Scholar]
- Wei, H.C.; Tye, L.; Bresnick, E.; Birt, D.F. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990, 50, 499–502. [Google Scholar]
- Basile, A.; Giordano, S.; Lopez-Saez, J.A.; Cobianchi, R.C. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar]
- Sato, Y.; Suzaki, S.; Nishikawa, T.; Kihara, M.; Shibata, H.; Higuti, T. Phytochemical flavones isolated from Scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2000, 72, 483–488. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, G.H.; Du, Y.G. Total synthesis of apigenin-4'-yl 2-O-(p-coumaroyl)-β-D-glucopyranoside. Carbohydr. Res. 2010, 345, 2714–2717. [Google Scholar] [CrossRef]
- Chen, P.; Han, J.J.; Liu, T.; Chen, L.Y. Synthesis of water-soluble apigenin. Food Sci. 2009, 30, 67–70. [Google Scholar]
- Zheng, X.; Meng, W.D.; Qing, F.L. Synthesis of gem-difluoromethylenated biflavonoid via the Suzuki coupling reaction. Tetrahedron Lett. 2004, 45, 8083–8085. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Botting, N.P. Synthesis of lupiwighteone via a para-Claisen–Cope rearrangement. Tetrahedron 2003, 59, 4177–4181. [Google Scholar] [CrossRef]
- Mavel, S.; Dikic, B.; Palakas, S.; Emond, P.; Greguric, I.; Gracia, A.G.; Mattner, F.; Garrigos, M.; Guilloteau, D.; Katsifis, A. Synthesis and biological evaluation of a series of flavone derivatives as potential radioligands for imaging the multidrug resistance-associated protein 1 (ABCC1/MRP1). Bioorg. Med. Chem. 2006, 14, 1599–1607. [Google Scholar] [CrossRef]
- Rasku, S.; Wähälä, K. Synthesis of deuterium labeled polyhydroxy flavones and 3-flavonols. Tetrahedron 2000, 56, 913–916. [Google Scholar] [CrossRef]
- Gunnarsson, G.T.; Riaz, M.; Adams, J.; Desai, U.R. Synthesis of per-sulfated flavonoids using 2,2,2-trichloroethyl protecting group and their factor Xa inhibition potential. Bioorg. Med. Chem. 2005, 13, 1783–1789. [Google Scholar] [CrossRef]
- Arend, M.; Westermann, B.; Risch, N. Modern Variants of the Mannich Reaction. Angew. Chem. Int. Ed. 1998, 37, 1044–1070. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Deng, L. The mannich reaction of malonates with simple imines catalyzed by bifunctional cinchona alkaloids: Enantioselective synthesis of β-amino acids. J. Am. Chem. Soc. 2006, 128, 6048–6049. [Google Scholar]
- Karthikeyan, M.S.; Prasad, D.J.; Poojary, B.; Bhat, K.S.; Holla, B.S.; Kumari, N.S. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg. Med. Chem. 2006, 14, 7482–7489. [Google Scholar]
- Holla, B.S.; Veerendra, B.; Shivananda, M.K.; Poojary, B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem. 2003, 38, 759–767. [Google Scholar] [CrossRef]
- Sujith, K.V.; Rao, J.N.; Shetty, P.; Kalluraya, B. Regioselective reaction: Synthesis and pharmacological study of Mannich bases containing ibuprofen moiety. Eur. J. Med. Chem. 2009, 44, 3697–3702. [Google Scholar] [CrossRef]
- Malinka, W.; Swiatek, P.; Filipek, B.; Sapa, J.; Jerierska, A.; Koll, A. Synthesis, analgesic activity and computational study of new isothiazolopyridines of Mannich base type. II Farmaco 2005, 60, 961–968. [Google Scholar] [CrossRef]
- Ashok, M.; Holla, B.S.; Poojary, B. Convenient one pot synthesis and antimicrobial evaluation of some new Mannich bases carrying 4-methylthiobenzyl moiety. Eur. J. Med. Chem. 2007, 42, 1095–1101. [Google Scholar] [CrossRef]
- Cogan, P.S.; Fowler, C.R.; Post, G.C.; Koch, T.H. Doxsaliform: A novel N-Mannich base prodrug of a doxorubicin formaldehyde conjugate. Lett. Drug Des. Discov. 2004, 1, 247–255. [Google Scholar]
- Babu, T.H.; Subba, R.V.R.; Tiwari, A.K.; Babu, K.S.; Srinivas, P.V.; Ali, A.Z.; Madhusudana, R.J. Synthesis and biological evaluation of novel 8-aminomethylated oroxylin A analogues as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 1659–1662. [Google Scholar]
- Zhang, S.X.; Ma, J.G.; Bao, Y.M.; Yang, P.W.; Zou, L.; Li, K.J.; Sun, X.D. Nitrogen-containing flavonoid analogues as CDK1/cyclin B inhibitors: Synthesis, SAR analysis, and biological activity. Bioorg. Med. Chem. 2008, 16, 7127–7132. [Google Scholar]
- Zhou, M.R.; Li, Y.; Dou, H.S.; Fan, C.H.; Gao, N.; Yin, S.F. Synthesis of derivatives of 8-aminomethylluteolin and their anti-inflammatory activity. Chem. Res. Appl. 2008, 20, 10–15. [Google Scholar]
- Dimmock, J.R.; Kandepu, N.M.; Hetherington, M.; Quail, J.W.; Pugazhenthi, U. Cytotoxic activities of Mannich bases of chalcones and related compounds. J. Med. Chem. 1998, 41, 1014–1026. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39–46. [Google Scholar] [CrossRef]
- Hassan Hilmy, K.M.; Khalifa, M.M.A.; Allah Hawata, M.A.; AboAlzeen Keshk, R.M.; El-Torgman, A.A. Synthesis of new pyrrolo[2,3-d]pyrimidine derivatives as antibacterial and antifungal agents. Eur. J. Med. Chem. 2010, 45, 5243–5250. [Google Scholar] [CrossRef]
- Lv, P.C.; Li, H.Q.; Xue, J.Y.; Shi, L.; Zhu, H.L. Synthesis and biological evaluation of novel luteolin derivatives as antibacterial agents. Eur. J. Med. Chem. 2009, 44, 908–914. [Google Scholar]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin. J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Cork, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar]
- Williams, W.B.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Hu, K.; Wang, W.; Ren, J. Synthesis and antitumor activities of mannich base derivatives of chrysin. J. Shenyang Pharm. Univ. 2010, 27, 448–452. [Google Scholar]
- Ren, J.; Pan, S.S.; Cheng, H.; Hu, K. Synthesis and antitumor activities of mannich base derivatives of luteolin. Chin. J. New Drugs 2011, 20, 743–747. [Google Scholar]
- Wang, X.B.; Liu, W.; Yang, L.; Guo, Q.L.; Kong, L.Y. Investigation on the substitution effects of the flavonoids as potent anticancer agents: a structure–activity relationships study. Med. Chem. Res. 2012, 21, 1833–1849. [Google Scholar]
- Li, J.J. Name Reactions a Collection of Detailed Reaction Mechanisms, 2nd ed; Springer: Berlin, Germany, 2003; pp. 246–247. [Google Scholar]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar]
- Jorgensen, J.H.; Crawford, S.A.; McElmeel, M.L.; Fiebelkorn, K.R. Detection of inducible clindamycin resistance of Staphylococci in conjunction with performance of automated broth susceptibility testing. J. Clin. Microbiol. 2004, 42, 1800–1802. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards (NCCLS), Performance Standards for Antimicrobial Disk Susceptibility Tests, 7th ed; NCCLS: Wayne, PA, USA, 2000; Approved standard, M2–A7.
- National Committee for Clinical Laboratory Standards (NCCLS), Performance Standards for Antimicrobial Susceptibility Testing; Document M100–S12; NCCLS: Wayne, PA, USA, 2002.
- Liu, Z.Q. Chemical methods to evaluate antioxidant ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar]
- Liu, J.Y.; Pang, Y.; Chen, J.; Huang, P.; Huang, W.; Zhu, X.Y.; Yan, D.Y. Hyperbranched polydiselenide as a self assembling broad spectrum anticancer agent. Biomaterials 2012, 33, 7765–7774. [Google Scholar]
- Xie, J.H.; Liu, X.; Shen, M.Y.; Nie, S.P.; Zhang, H.; Li, C.; Gong, D.M.; Xie, M.Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar]
- Sample Availability: Contact the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, R.; Zhao, B.; Wang, D.-E.; Yao, T.; Pang, L.; Tu, Q.; Ahmed, S.M.; Liu, J.-J.; Wang, J. Nitrogen-Containing Apigenin Analogs: Preparation and Biological Activity. Molecules 2012, 17, 14748-14764. https://doi.org/10.3390/molecules171214748
Liu R, Zhao B, Wang D-E, Yao T, Pang L, Tu Q, Ahmed SM, Liu J-J, Wang J. Nitrogen-Containing Apigenin Analogs: Preparation and Biological Activity. Molecules. 2012; 17(12):14748-14764. https://doi.org/10.3390/molecules171214748
Chicago/Turabian StyleLiu, Rui, Bin Zhao, Dong-En Wang, Tianyu Yao, Long Pang, Qin Tu, Saeed Mahmoud Ahmed, Jian-Jun Liu, and Jinyi Wang. 2012. "Nitrogen-Containing Apigenin Analogs: Preparation and Biological Activity" Molecules 17, no. 12: 14748-14764. https://doi.org/10.3390/molecules171214748
APA StyleLiu, R., Zhao, B., Wang, D.-E., Yao, T., Pang, L., Tu, Q., Ahmed, S. M., Liu, J.-J., & Wang, J. (2012). Nitrogen-Containing Apigenin Analogs: Preparation and Biological Activity. Molecules, 17(12), 14748-14764. https://doi.org/10.3390/molecules171214748