Hydroxysafflor Yellow A (HSYA) from Flowers of Carthamus tinctorius L. and Its Vasodilatation Effects on Pulmonary Artery
Abstract
:1. Introduction
2. Results and Discussion
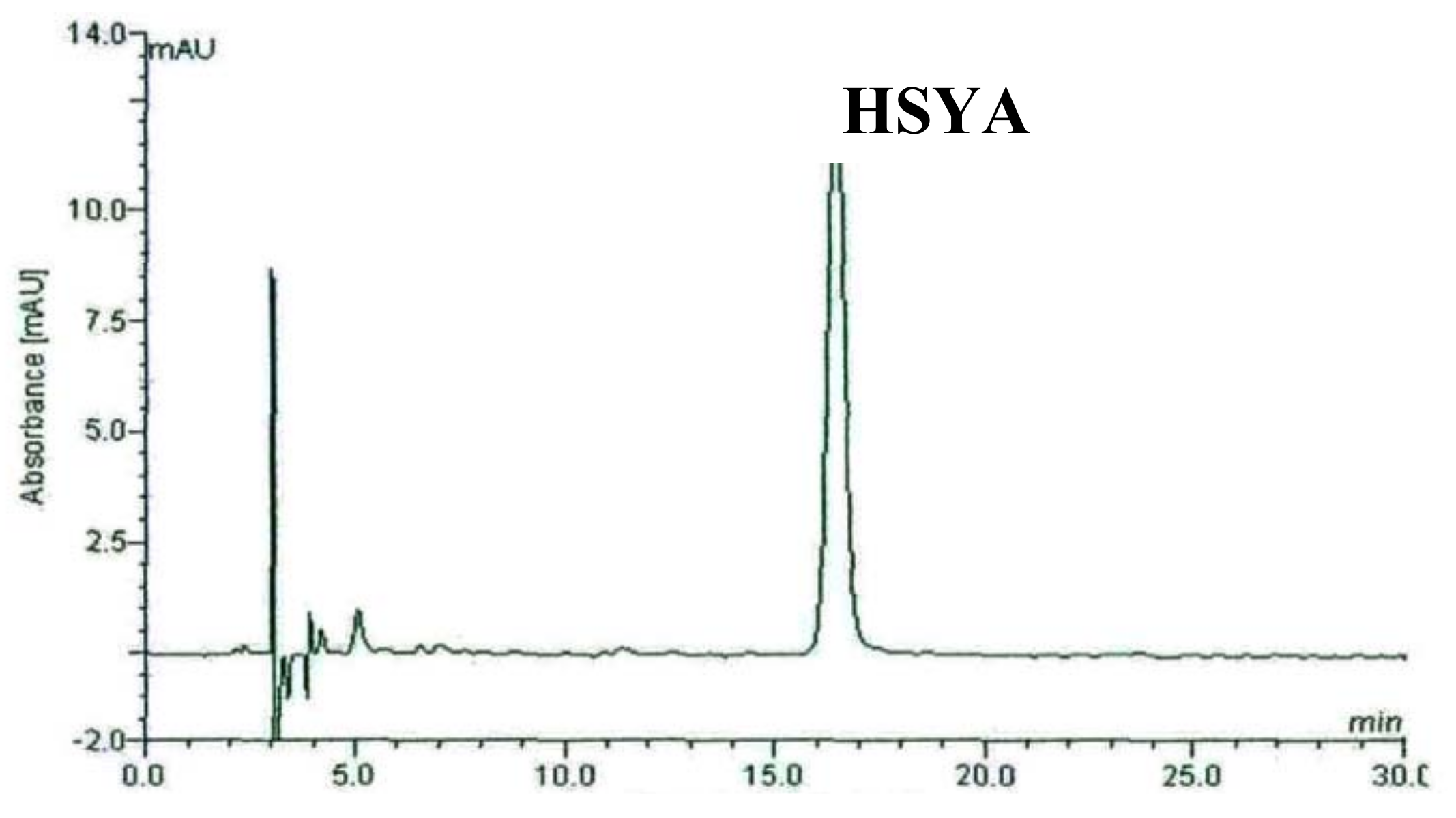
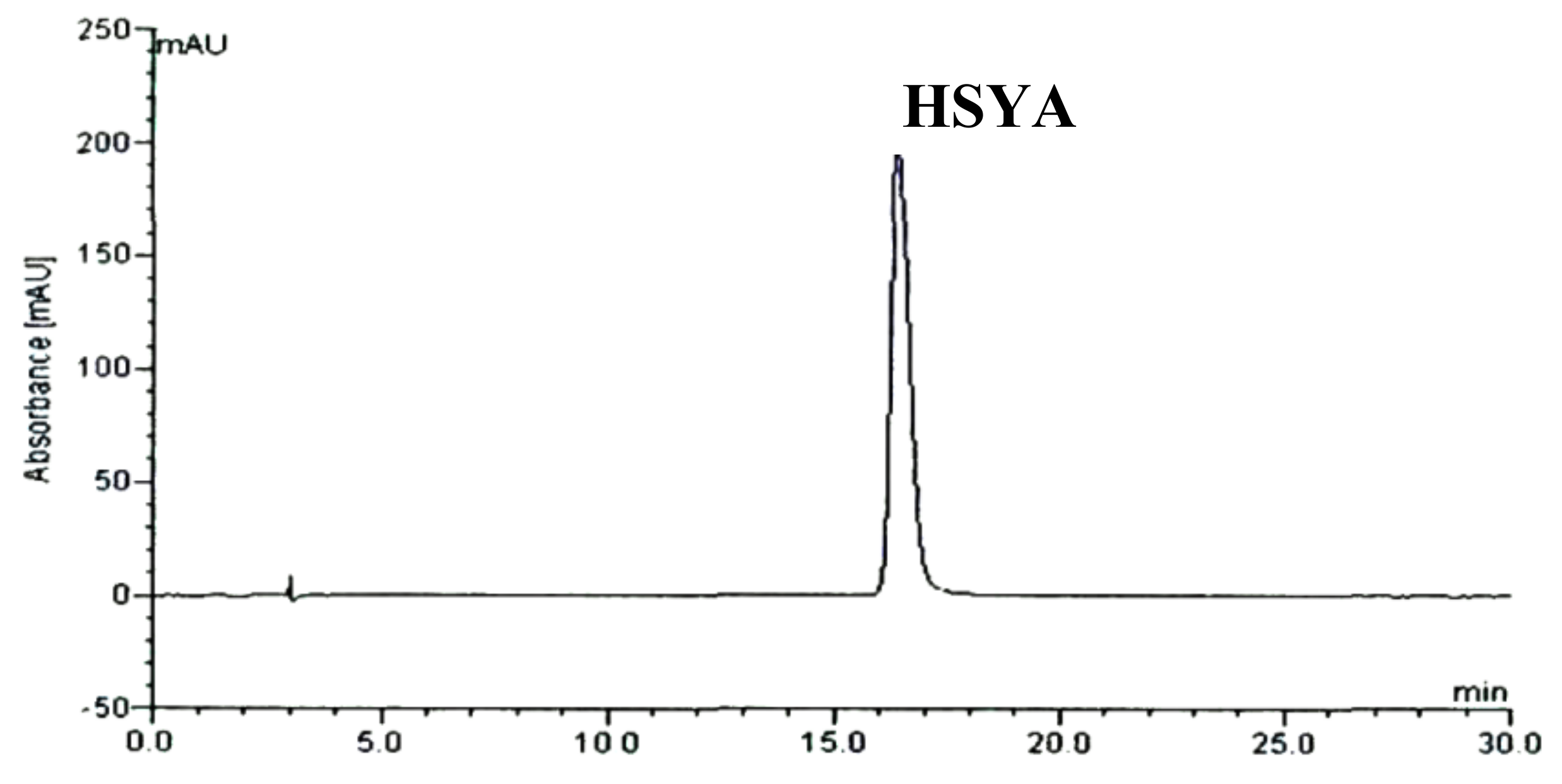
2.1. HPLC Chromatography of F2 from Carthamus tinctorius L. Crude Extract and Separated HSYA
2.2. Effects on PA Rings
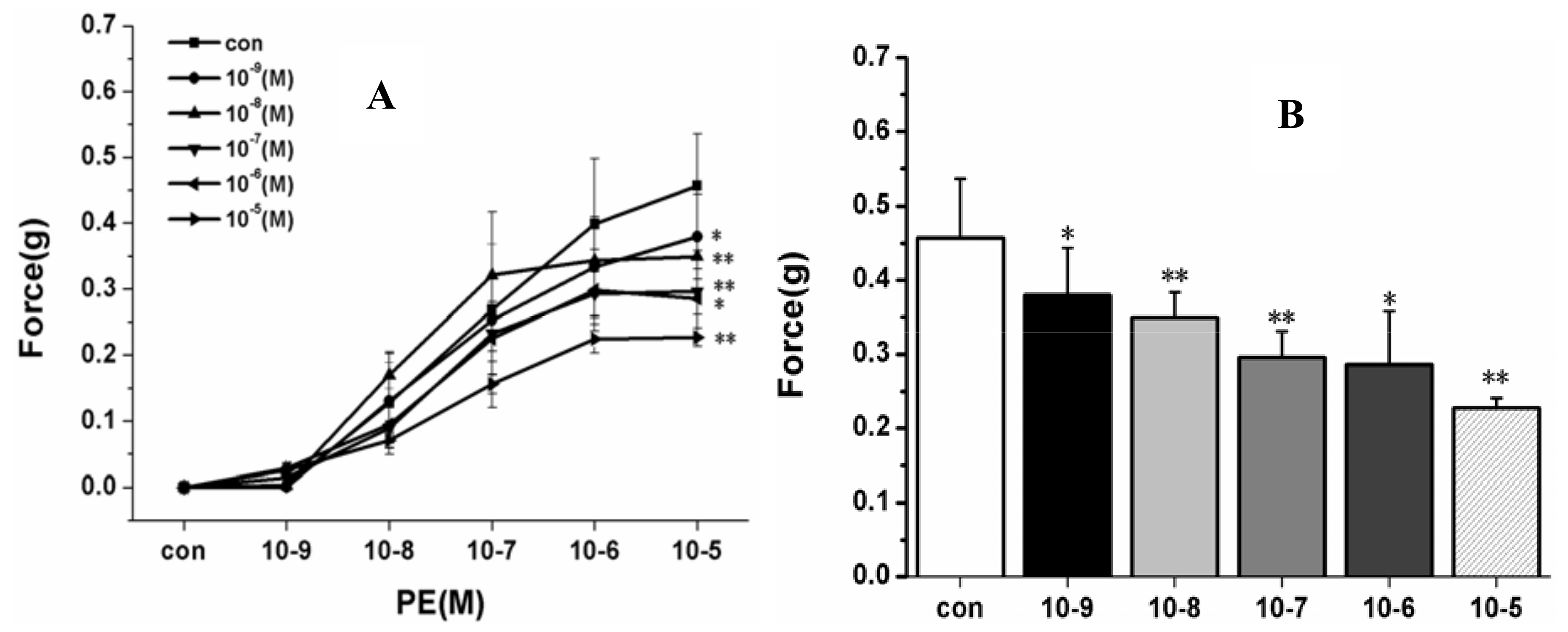
2.2.1. Effect of HSYA (10−9–10−5 M) on PE-Induced Vascular Constriction
2.2.2. Effect on Endothelium-Intact PA Rings
2.2.3. Effect on Endothelium-Denuded PA Rings
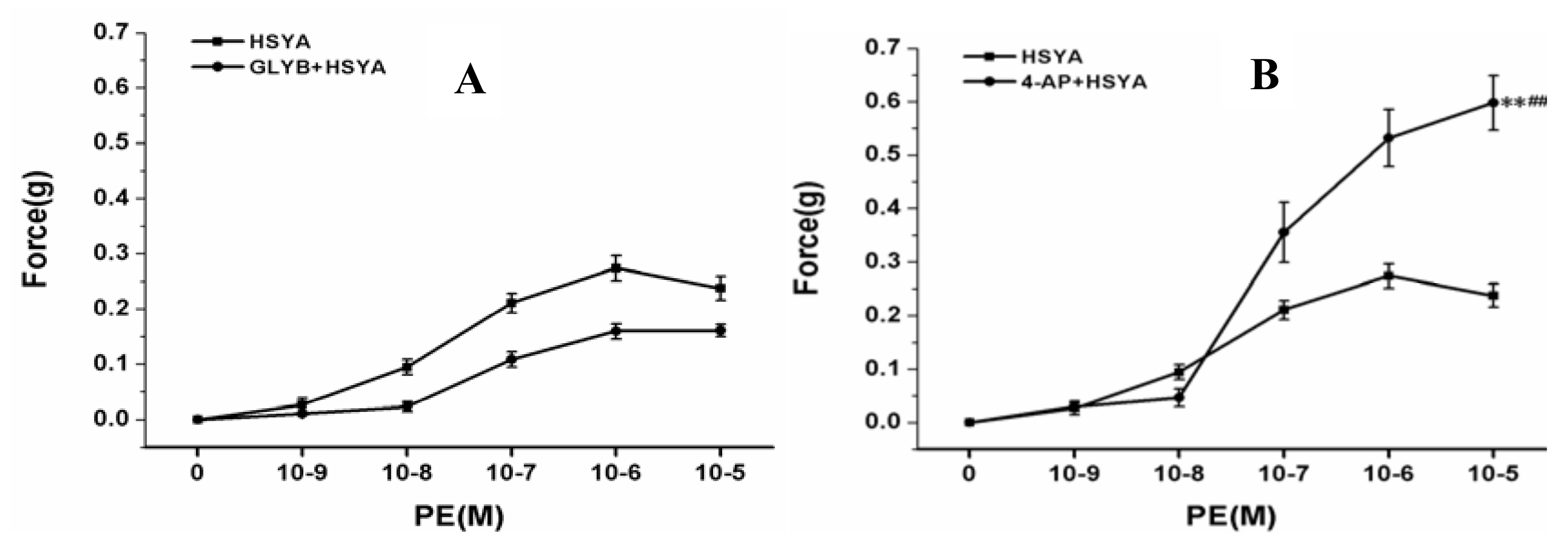
2.2.4. Effect on PA Rings after Blocking K+ Channels of PVSMCs
3. Experimental
3.1. Apparatus
3.2. Reagents and Materials
3.3. Plant Extract Preparation and Isolation of HSYA
3.4. Animals
3.5. Tension Studies of Pulmonary Arterial (PA) Rings
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Barst, R.J.; McGoon, M.; Torbicki, A.; Sitbon, O.; Krowka, M.J.; Olschewski, H.; Gaine, S. Diagnosis and differential assessment of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43, 40S–47S. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar] [PubMed]
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010, 122, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Black, C.M.; Keogh, A.; Pulido, T.; Frost, A.; Roux, S.; Leconte, I.; Landzberg, M. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2002, 346, 896–903. [Google Scholar]
- Galiè, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Tapson, V.F.; Gomberg-Maitland, M.; McLaughlin, V.V.; Benza, R.L.; Widlitz, A.C.; Krichman, A.; Barst, R.J. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest 2006, 129, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Committee of National Pharmacopoeia. P Harmacopoeia of People’s Republic of China (I); Chemical Industry Press: Beijing, China, 2010; pp. 141–142. [Google Scholar]
- Pu, Y.; Jin, M. Study of hydroxysafflower yellow A improves myocardial ischemia. Chin. Herbal. Med. 2005, 35, 374–475. [Google Scholar]
- Wang, Y.P.; Qiao, J.; Qian, Z.X. Studies on effect of safflower to protect ischemia of myocardium and antioxidation. Chin. J. Thorac. Cardiovasc. Surg. 1995, 11, 176–179. [Google Scholar]
- Wei, X.B.; Liu, H.Q.; Sun, X.; Fu, F.; Zhang, X.; Wang, J.; An, J.; Ding, H. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci. Lett. 2005, 386, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zang, B.; Jin, M.; Si, N.; Zhang, Y.; Wu, W.; Piao, Y.Z. Platelet activating factor receptor binding can be antagonized by HSYA. Acta Pharm. Sin. 2002, 37, 696–699. [Google Scholar]
- Wang, T.; Fu, F.; Han, B. Mechanism of Hydroxysafflor yellow A on protecting ischemic myocardium. J. Mol. Cell Cardiol. 2006, 40, 957. [Google Scholar] [CrossRef]
- Liu, F.; Wei, Y.; Yang, X. Hypotensive effects of safflower yellow in spontaneously hypertensive rats and influence on plasma renin activity and angiotensinII level. Acta Pharm. Sin. 1992, 27, 785–787. [Google Scholar]
- Tian, J.; Jang, W.; Wang, Z. Effects of safflower flavone on local cerebral ischemia and thrombosis formation in rats. Chin. Trad. Herb. Drugs 2003, 34, 741–743. [Google Scholar]
- Nie, P.H.; Zhang, L.; Zhang, W.H.; Rong, W.F.; Zhi, J.M. The effects of hydroxysafflor yellow A on blood pressure and cardiac function. J. Ethnopharmacol. 2012, 139, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.B.; Zhang, L.Y.; Li, C.L.; Ye, J.; Zhu, H.B. Effect of Hydroxysafflor yellow A on human umbilical vein endothelial cells under hypoxia. Vasc. Pharmacol. 2009, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, G.S.H.; Zhang, J.N.; Nie, P.H.; Zhang, J.M. Vascular effect of hydroxyl safflor yellow A and underlying mechanism. J. Shanghai Jiaotong Univ. (Med. Sci.) 2009, 29, 431–434. [Google Scholar]
- Meselhy, M.R.; Kadota, S.; Momose, Y.; Hatakeyama, N.; Kusai, A.; Hattori, M.; Namba, T. Two new quinochalcone yllow pigments from Carthamus tinctorius and Ca2+ antagonistic activity of tinctormine. Chem. Pharm. 1993, 41, 1796–1802. [Google Scholar] [CrossRef]
- Post, J.M.; Gelband, C.H.; Hume, J.R. [Ca2+]i inhibition of K+ channels in canine pulmonary artery Novel mechanism for hypoxia-induced membrane depolarization. Circ. Res. 1995, 77, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Post, J.M.; Hume, J.R.; Archer, S.L.; Weir, E.K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am. J. Physiol. 1992, 262, C882–C890. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Goldman, W.F.; Tod, M.L.; Rubin, L.J.; Blaustein, M.P. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am. J. Physiol. 1993, 264, L116–L123. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Huang, J.M.; Reeve, H.L. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ. Res. 1996, 78, 431–442. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the HSYA are available from the authors. |



© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bai, Y.; Lu, P.; Han, C.; Yu, C.; Chen, M.; He, F.; Yi, D.; Wu, L. Hydroxysafflor Yellow A (HSYA) from Flowers of Carthamus tinctorius L. and Its Vasodilatation Effects on Pulmonary Artery. Molecules 2012, 17, 14918-14927. https://doi.org/10.3390/molecules171214918
Bai Y, Lu P, Han C, Yu C, Chen M, He F, Yi D, Wu L. Hydroxysafflor Yellow A (HSYA) from Flowers of Carthamus tinctorius L. and Its Vasodilatation Effects on Pulmonary Artery. Molecules. 2012; 17(12):14918-14927. https://doi.org/10.3390/molecules171214918
Chicago/Turabian StyleBai, Yuhua, Ping Lu, Chenghua Han, Chunyue Yu, Minggang Chen, Fa He, Dan Yi, and Lijun Wu. 2012. "Hydroxysafflor Yellow A (HSYA) from Flowers of Carthamus tinctorius L. and Its Vasodilatation Effects on Pulmonary Artery" Molecules 17, no. 12: 14918-14927. https://doi.org/10.3390/molecules171214918



