Multicomponent Synthesis of 3,6-Dihydro-2H-1,3-thiazine-2-thiones
Abstract
:1. Introduction
2.1. Mechanistic Considerations

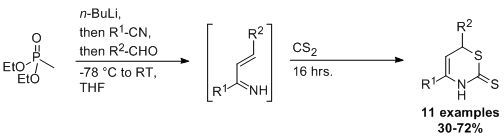
2. Results and Discussion

2.1. Scope
 | ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yield |
| 1 | Ph | Ph | 1a | 63% [a] |
| 2 | Ph | Ph | 1a | 69% [b] |
| 3 | iPr | Ph | 1b | 37% [b] |
| 4 | Ph | iPr | 1c | 38% [b] |
| 5 | 2,6-Me2C6H3 | 2,4,6-Me3C6H2 | 1d | 0% [b] |
| 6 | Ph | 2,4,6-Me3C6H2 | 1e | 30% [b] |
| 7 | p-F3CC6H4 | o- F3CC6H4 | 1f | n.d. [c] |
| 8 | p-F3CC6H4 | p- MeOC6H4 | 1g | 65% [d] |
| 9 | p-MeOC6H4 | o- F3CC6H4 | 1h | 59% [a] |
| 10 | p-MeOC6H4 | p- MeOC6H4 | 1i | 64% [b] |
| 11 | p- MeOC6H4 | o- MeOC6H4 | 1j | 65% [a] |
| 12 | p-BrC6H4 | p-BrC6H4 | 1k | 60% [b] |
| 13 | o-BrC6H4 | m-BrC6H4 | 1l | 72% [b] |
3. Experimental
3.1. General Information
4.2. General Procedure I for the Formation of 3,6-Dihydro-2H-1,3-thiazine-2-thiones
 According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv), benzonitrile (225 μL, 2.20 mmol), benzaldehyde (224 μL, 2.20 mmol) and carbon disulfide and subsequent flash column chromatography (EtOAc/c-hex = 1:15) afforded 1a as an orange foam (390 mg, 1.38 mmol, 69%). Alternatively, recrystrallization from MeOH afforded 1a as a light orange solid (356 mg, 1.26 mmol, 63%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.07 (bs, 1H), 7.51–7.43 (m, 5H), 7.41–7.31 (m, 1H), 5.62 (d, J = 5 Hz, 1H), 5.09 (d, J = 5 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 193.5 (C), 139.7 (C), 138.7 (C), 134.6 (C), 130.0 (CH), 129.3 (2 × CH), 129.2 (2 × CH), 128.5 (CH), 128.0 (2 × CH), 126.0 (2 × CH), 103.4 (CH), 46.8 (CH); HRMS [24] [MH+] 284.0538 (calc. C16H14NS2, 284.0562) [MH+−CS2] 208.1108 (calc. C15H14N, 208.1121); Melting point: 125–128 °C (decomp.).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv), benzonitrile (225 μL, 2.20 mmol), benzaldehyde (224 μL, 2.20 mmol) and carbon disulfide and subsequent flash column chromatography (EtOAc/c-hex = 1:15) afforded 1a as an orange foam (390 mg, 1.38 mmol, 69%). Alternatively, recrystrallization from MeOH afforded 1a as a light orange solid (356 mg, 1.26 mmol, 63%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.07 (bs, 1H), 7.51–7.43 (m, 5H), 7.41–7.31 (m, 1H), 5.62 (d, J = 5 Hz, 1H), 5.09 (d, J = 5 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 193.5 (C), 139.7 (C), 138.7 (C), 134.6 (C), 130.0 (CH), 129.3 (2 × CH), 129.2 (2 × CH), 128.5 (CH), 128.0 (2 × CH), 126.0 (2 × CH), 103.4 (CH), 46.8 (CH); HRMS [24] [MH+] 284.0538 (calc. C16H14NS2, 284.0562) [MH+−CS2] 208.1108 (calc. C15H14N, 208.1121); Melting point: 125–128 °C (decomp.). According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), isobutyronitrile (197 μL, 2.20 mmol), benzaldehyde (224 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1b as a yellow solid (185 mg, 0.74 mmol, 37%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 8.62 (bs, 1H), 7.39–7.24 (m, 5H), 5.15 (d, J = 9.5 Hz, 1H), 4.88 (d, J = 9.5 Hz, 1H), 2.46 (sept, J = 7 Hz, 1H), 1.22 (d, J = 7 Hz, 3H), 1.21 (d, J = 7Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 193.3 (C), 143.6 (C), 140.5 (C), 129.1 (2 × CH), 128.3 (CH), 127.8 (2 × CH), 99.6 (CH), 46.3 (CH), 32.4 (CH), 20.8 (CH3), 20.7 (CH3); HRMS [MH+] 250.0712 (calc. C13H16NS2, 250.0719) [MH+−CS2] 174.1275 (calc. C12H16N, 174.1277); Melting point: 94–95 °C.
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), isobutyronitrile (197 μL, 2.20 mmol), benzaldehyde (224 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1b as a yellow solid (185 mg, 0.74 mmol, 37%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 8.62 (bs, 1H), 7.39–7.24 (m, 5H), 5.15 (d, J = 9.5 Hz, 1H), 4.88 (d, J = 9.5 Hz, 1H), 2.46 (sept, J = 7 Hz, 1H), 1.22 (d, J = 7 Hz, 3H), 1.21 (d, J = 7Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 193.3 (C), 143.6 (C), 140.5 (C), 129.1 (2 × CH), 128.3 (CH), 127.8 (2 × CH), 99.6 (CH), 46.3 (CH), 32.4 (CH), 20.8 (CH3), 20.7 (CH3); HRMS [MH+] 250.0712 (calc. C13H16NS2, 250.0719) [MH+−CS2] 174.1275 (calc. C12H16N, 174.1277); Melting point: 94–95 °C. According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), benzonitrile (225 μL, 2.20 mmol), isobutyraldehyde (200 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1c as a light yellow solid (192 mg, 0.77 mmol, 38%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 8.91 (bs, 1H), 7.43 (s, 5H), 5.45 (d, J = 5 Hz), 3.68 (t, J = 7.5 Hz, 1H), 2.03 (m, 1H), 1.07 (d, J = 6.5 Hz, 3H), 1.06 (d, J = 6.5 Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 194.9 (C), 138.4 (C), 134.9 (C), 129.8 (CH), 129.2 (2 × CH), 125.9 (2 × CH), 102.6 (CH), 49.7 (CH), 34.7 (CH), 18.9 (CH3), 18.6 (CH3); HRMS [MH+] 250.0710 (calc. C13H16NS2, 250.0719) [MH+−CS2] 174.1273 (calc. C12H16N, 174.1277); Melting point: 93–95 °C.
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), benzonitrile (225 μL, 2.20 mmol), isobutyraldehyde (200 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1c as a light yellow solid (192 mg, 0.77 mmol, 38%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 8.91 (bs, 1H), 7.43 (s, 5H), 5.45 (d, J = 5 Hz), 3.68 (t, J = 7.5 Hz, 1H), 2.03 (m, 1H), 1.07 (d, J = 6.5 Hz, 3H), 1.06 (d, J = 6.5 Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 194.9 (C), 138.4 (C), 134.9 (C), 129.8 (CH), 129.2 (2 × CH), 125.9 (2 × CH), 102.6 (CH), 49.7 (CH), 34.7 (CH), 18.9 (CH3), 18.6 (CH3); HRMS [MH+] 250.0710 (calc. C13H16NS2, 250.0719) [MH+−CS2] 174.1273 (calc. C12H16N, 174.1277); Melting point: 93–95 °C. According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), benzonitrile (225 μL, 2.20 mmol), mesitylene carboxaldehyde (324 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1e as a yellow foam (194 mg, 0.60 mmol, 30%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.02 (bs, 1H), 7.48–7.42 (m, 5H), 6.89 (s, 2H), 5.88 (d, J = 3.5, 1H), 5.54 (d, J = 3.5 Hz, 1H), 2.45 (bs, 6H), 2.28 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 196.6 (C), 138.3 (3 × C), 137.5 (C), 134.6 (C), 129.9 (C), 129.8 (2 × CH), 129.3 (3 × CH), 125.8 (2 × CH), 105.6 (CH), 42.6 (CH), 20.9 (3 × CH3); HRMS [MH+] 326.1020 (calc. C19H20NS2, 326.1032) [MH+−CS2] 250.1585 (calc. C18H20N, 250.1590).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), benzonitrile (225 μL, 2.20 mmol), mesitylene carboxaldehyde (324 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1e as a yellow foam (194 mg, 0.60 mmol, 30%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.02 (bs, 1H), 7.48–7.42 (m, 5H), 6.89 (s, 2H), 5.88 (d, J = 3.5, 1H), 5.54 (d, J = 3.5 Hz, 1H), 2.45 (bs, 6H), 2.28 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 196.6 (C), 138.3 (3 × C), 137.5 (C), 134.6 (C), 129.9 (C), 129.8 (2 × CH), 129.3 (3 × CH), 125.8 (2 × CH), 105.6 (CH), 42.6 (CH), 20.9 (3 × CH3); HRMS [MH+] 326.1020 (calc. C19H20NS2, 326.1032) [MH+−CS2] 250.1585 (calc. C18H20N, 250.1590). According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-trifluoromethylbenzonitrile (376 mg, 2.20 mmol), o-trifluoromethylbenzaldehyde (290 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded an inseparable mixture of 1f and impurities (367 mg) 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.12 (bs, 1H), 8.10–7.20 (m, 8H), 5.60 (d, J = 5 Hz, 1H), 5.47 (d, J = 5 Hz, 1H); HRMS [MH+] 420.0255 (calc. C18H12F6NS2, 420.0310) [MH+−CS2] 344.0847 (calc. C17H12F6N, 344.0868).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-trifluoromethylbenzonitrile (376 mg, 2.20 mmol), o-trifluoromethylbenzaldehyde (290 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded an inseparable mixture of 1f and impurities (367 mg) 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.12 (bs, 1H), 8.10–7.20 (m, 8H), 5.60 (d, J = 5 Hz, 1H), 5.47 (d, J = 5 Hz, 1H); HRMS [MH+] 420.0255 (calc. C18H12F6NS2, 420.0310) [MH+−CS2] 344.0847 (calc. C17H12F6N, 344.0868). According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-trifluoromethylbenzonitrile (376 mg, 2.20 mmol), p-methoxybenzaldehyde (335 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded an inseparable mixture of 1g and impurities (599 mg). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.10 (bs, 1H), 7.71 (d, J = 8 Hz, 2H), 7.61 (d, J = 8 Hz, 2H), 7.28 (d, J = 9 Hz, 2H), 6.90 (d, J = 9 Hz, 2H), 5.67 (d, J = 4.5 Hz, 1H), 5.47 (d, J = 4.5 Hz, 1H), 3.81 (s, 3H); HRMS [MH+] 382.0515 (calc. C18H15F3NOS2, 382.0542) [MH+−CS2] 306.1085 (calc. C17H15F3NO, 306.1100).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-trifluoromethylbenzonitrile (376 mg, 2.20 mmol), p-methoxybenzaldehyde (335 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded an inseparable mixture of 1g and impurities (599 mg). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.10 (bs, 1H), 7.71 (d, J = 8 Hz, 2H), 7.61 (d, J = 8 Hz, 2H), 7.28 (d, J = 9 Hz, 2H), 6.90 (d, J = 9 Hz, 2H), 5.67 (d, J = 4.5 Hz, 1H), 5.47 (d, J = 4.5 Hz, 1H), 3.81 (s, 3H); HRMS [MH+] 382.0515 (calc. C18H15F3NOS2, 382.0542) [MH+−CS2] 306.1085 (calc. C17H15F3NO, 306.1100). According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-methoxybenzonitrile (293 mg, 2.20 mmol), o-trifluoromethylbenzaldehyde (290 μL, 2.20 mmol) and carbon disulfide and subsequent recrystallization from MeOH afforded 1h as a light yellow solid (448 mg, 1.18 mmol, 59%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.09 (bs, 1H), 7.71 (d, J = 8 Hz, 1H), 7.67 (d, J = 8 Hz, 1H), 7.60 (t, J = 7.5 Hz), 7.46-7.38 (m, 3H), 6.95 (d, J = 9 Hz, 2H), 5.44 (d, J = 5 Hz, 1H), 5.41 (d, J = 5 Hz, 1H), 3.84 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 192.4 (C), 161.0 (C), 139.5 (C), 138.2 (C), 133.0 (CH), 130.8 (CH), 127.4 (q, J = 18 Hz, C), 127.4 (2 × CH), 126.0 (q, J = 6 Hz, CH), 125.1 (CH) 123.5 (q, J = 403 Hz, CF3) 114.6 (2 × CH), 101.1 (CH), 55.5 (CH3), 42.2 (CH); HRMS [MH+] 382.0523 (calc. C18H15F3NOS2, 382.0542) [MH+−CS2] 306.1084 (calc. C17H15F3NO, 306.1100); Melting point: 147–148 °C (decomp.).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-methoxybenzonitrile (293 mg, 2.20 mmol), o-trifluoromethylbenzaldehyde (290 μL, 2.20 mmol) and carbon disulfide and subsequent recrystallization from MeOH afforded 1h as a light yellow solid (448 mg, 1.18 mmol, 59%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.09 (bs, 1H), 7.71 (d, J = 8 Hz, 1H), 7.67 (d, J = 8 Hz, 1H), 7.60 (t, J = 7.5 Hz), 7.46-7.38 (m, 3H), 6.95 (d, J = 9 Hz, 2H), 5.44 (d, J = 5 Hz, 1H), 5.41 (d, J = 5 Hz, 1H), 3.84 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 192.4 (C), 161.0 (C), 139.5 (C), 138.2 (C), 133.0 (CH), 130.8 (CH), 127.4 (q, J = 18 Hz, C), 127.4 (2 × CH), 126.0 (q, J = 6 Hz, CH), 125.1 (CH) 123.5 (q, J = 403 Hz, CF3) 114.6 (2 × CH), 101.1 (CH), 55.5 (CH3), 42.2 (CH); HRMS [MH+] 382.0523 (calc. C18H15F3NOS2, 382.0542) [MH+−CS2] 306.1084 (calc. C17H15F3NO, 306.1100); Melting point: 147–148 °C (decomp.). According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-methoxybenzonitrile (393 mg, 2.20 mmol), p-methoxybenzaldehyde (335 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1i as an orange foam (441 mg, 1.28 mmol, 64%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.04 (bs, 1H), 7.40 (d, J = 9 Hz, 2H), 7.28 (d, J = 9 Hz, 2H), 6.95 (d, J = 9 Hz, 2H), 6.89 (d, J = 9 Hz, 2H), 5.50 (d, J = 5 Hz, 1H), 5.04 (d, J = 5 Hz, 1H), 3.84 (s, 3H), 3.80 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 193.7 (C), 160.9 (C), 159.7 (C), 138.3 (C), 131.7 (C), 129.2 (2 × CH), 127.4 (2 × CH), 127.0 (C), 114.6 (2 × CH), 114.5 (2 × CH), 102.5, (CH), 55.5 (CH3), 55.4 (CH3), 46.4 (CH); HRMS [MH+] 344.0741 (calc. C18H18NO2S2, 344.0773) [MH+−CS2] 268.1310 (calc. C17H18NO2, 268.1332).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-methoxybenzonitrile (393 mg, 2.20 mmol), p-methoxybenzaldehyde (335 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1i as an orange foam (441 mg, 1.28 mmol, 64%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.04 (bs, 1H), 7.40 (d, J = 9 Hz, 2H), 7.28 (d, J = 9 Hz, 2H), 6.95 (d, J = 9 Hz, 2H), 6.89 (d, J = 9 Hz, 2H), 5.50 (d, J = 5 Hz, 1H), 5.04 (d, J = 5 Hz, 1H), 3.84 (s, 3H), 3.80 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 193.7 (C), 160.9 (C), 159.7 (C), 138.3 (C), 131.7 (C), 129.2 (2 × CH), 127.4 (2 × CH), 127.0 (C), 114.6 (2 × CH), 114.5 (2 × CH), 102.5, (CH), 55.5 (CH3), 55.4 (CH3), 46.4 (CH); HRMS [MH+] 344.0741 (calc. C18H18NO2S2, 344.0773) [MH+−CS2] 268.1310 (calc. C17H18NO2, 268.1332). According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-methoxybenzonitrile (393 mg, 2.20 mmol), o-methoxybenzaldehyde (300 mg, 2.20 mmol) and carbon disulfide and subsequent recrystallization from MeOH afforded 1j as a light orange solid (448 mg, 1.30 mmol, 65%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.00 (bs, 1H), 7.43 (d, J = 8 Hz, 2H, 7.33–7.25 (m, 2H), 6.98–6.93 (m, 3H), 6.90 (d, J = 8 Hz, 1H), 5.50 (d, J = 6 Hz, 1H), 5.36 (d, J = 6 Hz, 1H), 3.87 (s, 3H), 3.85 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 194.3 (C), 160.8 (C), 156.4 (C), 138.8 (C), 129.4 (CH), 128.2 (CH), 127.4 (2 × CH), 127.2 (C), 120.9 (CH), 114.6 (2 × CH), 110.8 (CH), 100.8 (CH), 55.6 (CH3), 55.5 (CH3), 40.0 (CH); HRMS [MH+] 344.0755 (calc. C18H18NO2S2, 344.0773) [MH+−CS2] 268.1318 (calc. C17H18NO2, 268.1332); Melting point: 139–143 °C (decomp.).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.5 mL, 2.4 mmol, 1.2 equiv.), p-methoxybenzonitrile (393 mg, 2.20 mmol), o-methoxybenzaldehyde (300 mg, 2.20 mmol) and carbon disulfide and subsequent recrystallization from MeOH afforded 1j as a light orange solid (448 mg, 1.30 mmol, 65%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.00 (bs, 1H), 7.43 (d, J = 8 Hz, 2H, 7.33–7.25 (m, 2H), 6.98–6.93 (m, 3H), 6.90 (d, J = 8 Hz, 1H), 5.50 (d, J = 6 Hz, 1H), 5.36 (d, J = 6 Hz, 1H), 3.87 (s, 3H), 3.85 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 194.3 (C), 160.8 (C), 156.4 (C), 138.8 (C), 129.4 (CH), 128.2 (CH), 127.4 (2 × CH), 127.2 (C), 120.9 (CH), 114.6 (2 × CH), 110.8 (CH), 100.8 (CH), 55.6 (CH3), 55.5 (CH3), 40.0 (CH); HRMS [MH+] 344.0755 (calc. C18H18NO2S2, 344.0773) [MH+−CS2] 268.1318 (calc. C17H18NO2, 268.1332); Melting point: 139–143 °C (decomp.). According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.3 mL, 2.1 mmol, 1.05 equiv.), p-bromobenzonitrile (400 mg, 2.20 mmol), p-bromobenzaldehyde (407 mg, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1k as a red foam (532 mg, 1.21 mmol, 60%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.02 (bs, 1H), 7.58 (d, J = 6.5 Hz, 2H), 7.50 (d, J = 6.5 Hz, 2H), 7.34 (d, J = 6.5 Hz, 2H), 7.22 (d, J = 6.5 Hz, 2H), 5.57 (d, J = 5 Hz, 1H), 5.01 (d, J = 5Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 193.0 (C), 138.6 (C), 138.1 (C), 133.3 (C), 132.5 (2 × CH), 132.4 (2 × CH), 129.5 (2 × CH), 127.6 (2 × CH), 124.4 (C), 122.7 (C), 103.2 (CH), 46.1 (CH); HRMS [MNa+] 461.8557 (calc. C19H11Br2NNaS2, 461.8592) [MH+−CS2] 363.9304 (calc. C15H12Br2N, 363.9331).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.3 mL, 2.1 mmol, 1.05 equiv.), p-bromobenzonitrile (400 mg, 2.20 mmol), p-bromobenzaldehyde (407 mg, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1k as a red foam (532 mg, 1.21 mmol, 60%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.02 (bs, 1H), 7.58 (d, J = 6.5 Hz, 2H), 7.50 (d, J = 6.5 Hz, 2H), 7.34 (d, J = 6.5 Hz, 2H), 7.22 (d, J = 6.5 Hz, 2H), 5.57 (d, J = 5 Hz, 1H), 5.01 (d, J = 5Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 193.0 (C), 138.6 (C), 138.1 (C), 133.3 (C), 132.5 (2 × CH), 132.4 (2 × CH), 129.5 (2 × CH), 127.6 (2 × CH), 124.4 (C), 122.7 (C), 103.2 (CH), 46.1 (CH); HRMS [MNa+] 461.8557 (calc. C19H11Br2NNaS2, 461.8592) [MH+−CS2] 363.9304 (calc. C15H12Br2N, 363.9331). According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.3 mL, 2.1 mmol, 1.05 eq), o-bromobenzonitrile (400 mg, 2.20 mmol), m-bromobenzaldehyde (257 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1l as a light pink foam (638 mg, 1.45 mmol, 72%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 8.93 (bs, 1H), 7.66 (d, J = 8 Hz, 1H), 7.57 (s, 1H), 7.44 (d, J = 7.5, 1H), 7.38 (t, J = 3 Hz, 2H), 7.36-7.28 (m, 2H) 7.25 (t, J = 6 Hz, 1H), 5.42 (d, J = 5 Hz, 1H), 4.97 (d, J = 5 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 191.9 (C), 142.2 (C), 138.3 (C), 135.3 (C), 133.6 (CH), 131.6 (CH), 131.5 (CH), 131.3 (CH), 131.0 (CH), 130.7 (CH), 128.0 (CH), 126.6 (CH), 123.1 (C), 122.5 (C), 105.1 (CH), 46.1 (CH); HRMS [MNa+] 461.8569 (calc. C19H11Br2NNaS2, 461.8592) [MH+−CS2] 363.9309 (calc. C15H12Br2N, 363.9331).
According to General procedure I, the reaction between diethyl methylphosphonate, n-butyllithium (1.3 mL, 2.1 mmol, 1.05 eq), o-bromobenzonitrile (400 mg, 2.20 mmol), m-bromobenzaldehyde (257 μL, 2.20 mmol) and carbon disulfide followed by flash column chromatography (EtOAc/c-hex = 1:15) afforded 1l as a light pink foam (638 mg, 1.45 mmol, 72%). 1H-NMR (500 MHz, CDCl3) δ (ppm) 8.93 (bs, 1H), 7.66 (d, J = 8 Hz, 1H), 7.57 (s, 1H), 7.44 (d, J = 7.5, 1H), 7.38 (t, J = 3 Hz, 2H), 7.36-7.28 (m, 2H) 7.25 (t, J = 6 Hz, 1H), 5.42 (d, J = 5 Hz, 1H), 4.97 (d, J = 5 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ (ppm) 191.9 (C), 142.2 (C), 138.3 (C), 135.3 (C), 133.6 (CH), 131.6 (CH), 131.5 (CH), 131.3 (CH), 131.0 (CH), 130.7 (CH), 128.0 (CH), 126.6 (CH), 123.1 (C), 122.5 (C), 105.1 (CH), 46.1 (CH); HRMS [MNa+] 461.8569 (calc. C19H11Br2NNaS2, 461.8592) [MH+−CS2] 363.9309 (calc. C15H12Br2N, 363.9331).4. Conclusions
Supplementary Materials
Acknowledgments
Conflict of Interest
- Sample Availability: Samples of compound 1a are available from the authors.
References and Notes
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar]
- Groenendaal, B.; Vugts, D. J.; Schmitz, R.F.; de Kanter, F.J.J.; Ruijter, E.; Groen, M.B.; Orru, R.V.A. A multicomponent synthesis of triazinane diones. J. Org. Chem. 2008, 73, 719–722. [Google Scholar]
- Vugts, D.J.; Koningstein, M.M.; Schmitz, R.F.; de Kanter, F.J.J.; Groen, M.B.; Orru, R.V.A. Multicomponent synthesis of dihydropyrimidines and thiazines. Chem. Eur. J. 2006, 12, 7178–7189. [Google Scholar] [CrossRef]
- Scheffelaar, R.; Paravidino, M.; Znabet, A.; Schmitz, R.F.; de Kanter, F.J.J.; Lutz, M.; Spek, A.L.; Guerra, C.F.; Bickelhaupt, F.M.; Groen, M.B.; et al. Scope and limitations of an efficient four-component reaction for dihydropyridin-2-ones. J. Org. Chem. 2010, 75, 1723–1732. [Google Scholar]
- Kiselyov, A.S. One-pot synthesis of polysubstituted pyrimidines. Tetrahedron Lett. 2005, 46, 1663–1665. [Google Scholar]
- Groenendaal, B.; Ruijter, E.; Orru, R.V.A. 1-Azadienes in cycloaddition and multicomponent reactions towards N-heterocycles. Chem. Commun. 2008, 5474–5489. [Google Scholar]
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 3rd ed; Wiley-Interscience Publication: New York, NY, USA, 1999. [Google Scholar]
- Ryu, I.; Murai, S.; Sonoda, N. A novel synthesis of β-trichlorostannyl ketones from siloxycyclopropanes and their facile dehydrostannation affording 2-methylene ketones. J. Org. Chem. 1986, 51, 2389–2391. [Google Scholar]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process—A second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar]
- Hartung, J.; Schur, C.; Kempter, I.; Gottwald, T. Efficiency of alkoxyl radical product formation from 5-substituted 3-alkoxy-4-methylthiazole-2(3H)-thiones. Tetrahedron 2010, 66, 1365–1374. [Google Scholar] [CrossRef]
- Beckwith, A.L.J.; Schiesser, C.H. Treasures from the free radical renaissance period-Miscellaneous hexenyl radical kinetic data. Org. Biomol. Chem. 2011, 9, 1736–1743. [Google Scholar]
- Schur, C.; Becker, N.; Bergstraesser, U.; Hartung, J.; Gottwald, T. Tertiary alkoxyl radicals from 3-alkoxythiazole-2(3H)-thiones. Tetrahedron 2011, 67, 2338–2347. [Google Scholar]
- Alagarsamy, V.; Pathak, U.S. Synthesis and antihypertensive activity of novel 3-benzyl-2-substituted-3H-[1,2,4]triazolo[5,1-b]quinazolin-9-ones. Bioorg. Med. Chem. 2007, 15, 3457–3462. [Google Scholar]
- Das, P.; Kumar, C.K.; Kumar, K.N.; Innus, M.; Iqbal, J.; Srinivas, N. Dithiocarbamate and CuO promoted one-pot synthesis of 2-(N-substituted)-aminobenzimidazoles and related heterocycles. Tetrahedron Lett. 2008, 49, 992–995. [Google Scholar]
- Gobis, K.; Foks, H.; Zwolska, Z.; Augustynowicz-Kope, E. Synthesis and tuberculostatic activity of novel 1,2,4-triazoles obtained from heterocyclic carbohydrazides. Heterocycles 2010, 81, 917–934. [Google Scholar] [CrossRef]
- Shutalev, A.D.; Pagaev, M.T.; Ignatova, L.A. Addition of dithiocarbaminic acid to alpha,beta-unsaturated aldehydes and ketones—Simple synthesis of 4-alkoxytertrahydro-1,3-thiazine-2-thiones. Zh. Org. Khim. 1991, 27, 1274–1285. [Google Scholar]
- Jansen, J.E.; Mathes, R.A. Some thiazinethiols and their intermediate compounds. J. Am. Chem. Soc. 1955, 77, 2866. [Google Scholar]
- Arbusow, B.A.; Soroastrowa, V.M. Communication 2. Alkylation of some heterocyclic compounds by derivatives of phosphoric and phosphourous acids. Russ. Chem. Bull. 1959, 1001–1004. [Google Scholar]
- Garraway, J.L. Cyclic derivatives of dithiocarbamic acids. J. Chem. Soc. 1964, 760, 4008–4011. [Google Scholar] [CrossRef]
- Perjesi, P.; Szabo, D.; Foldesi, A. Synthesis of 4,6-diaryl-2,3-dihydro-6H-1,3-thiazine-2-thiones by the reaction of chalcones with dithiocarbamic acid. Acta Chim. Hung. 1986, 122, 119–125. [Google Scholar]
- Lee, K.; Oh, D.Y. One-pot synthesis of α,β-unsaturated ketones. Synthesis 1991, 1991, 213–214. [Google Scholar] [CrossRef]
- Shin, W.S.; Lee, K.; Oh, D.Y. One-pot synthesis of primary E-allylic amines. Tetrahedron Lett. 1995, 36, 281–282. [Google Scholar] [CrossRef]
- den Heeten, R.; van der Boon, L.J.P.; Broere, D.L.J.; Janssen, E.; de Kanter, F.J.J.; Ruijter, E.; Orru, R.V.A. Concise synthesis of highly substituted benzo[a]quinolizines by a multicomponent reaction/allylation/Heck reaction sequence. Eur. J. Org. Chem. 2012, 275–280. [Google Scholar]
- The base peak in all HRMS spectra represents the 1-azadiene, e.g., the product−CS2. This is most likely due to better ionizability of the 1-azadiene combined with the entropic benefit of the formal retro-hetero-Diels-Alder reaction under ESI conditions.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kruithof, A.; Ploeger, M.L.; Janssen, E.; Helliwell, M.; Kanter, F.J.J.d.; Ruijter, E.; Orru, R.V.A. Multicomponent Synthesis of 3,6-Dihydro-2H-1,3-thiazine-2-thiones. Molecules 2012, 17, 1675-1685. https://doi.org/10.3390/molecules17021675
Kruithof A, Ploeger ML, Janssen E, Helliwell M, Kanter FJJd, Ruijter E, Orru RVA. Multicomponent Synthesis of 3,6-Dihydro-2H-1,3-thiazine-2-thiones. Molecules. 2012; 17(2):1675-1685. https://doi.org/10.3390/molecules17021675
Chicago/Turabian StyleKruithof, Art, Marten L. Ploeger, Elwin Janssen, Madeleine Helliwell, Frans J. J. de Kanter, Eelco Ruijter, and Romano V. A. Orru. 2012. "Multicomponent Synthesis of 3,6-Dihydro-2H-1,3-thiazine-2-thiones" Molecules 17, no. 2: 1675-1685. https://doi.org/10.3390/molecules17021675