Synthesis, Characterization, X-ray Crystallography, Acetyl Cholinesterase Inhibition and Antioxidant Activities of Some Novel Ketone Derivatives of Gallic Hydrazide-Derived Schiff Bases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

2.2. X-ray Crystallography

| Distances | |
|---|---|
| N1-N2 | 1.375(4) |
| N1-C8 | 1.294(4) |
| N2-C10 | 1.374(4) |
| C10-O3 | 1.231(4) |
| Angles | |
| C8-N1-N2 | 121.0(3) |
| C10-N2-N1 | 116.6(3) |
| O3-C10-N2 | 120.5(3) |
| O2-C5-C6 | 123.0(3) |
| Identification code | 4 | 5 |
|---|---|---|
| Empirical formula | C16H18N2O7 | C14H12N3O5 |
| Formula weight | 350.32 | 302.27 |
| Temperature/K | 569(2) | 296 |
| Crystal system | Monoclinic | triclinic |
| Space group | P21/n | P-1 |
| a/Å | 7.769(9) | 6.6282(7) |
| b/Å | 15.509(19) | 9.9326(7) |
| c/Å | 13.162(16) | 12.8906(11) |
| α/° | 90 | 79.982(2) |
| β/° | 103.85(2) | 80.995(2) |
| γ/° | 90 | 76.5060(10) |
| Volume/Å3 | 1,540(3) | 806.67(12) |
| Z | 4 | 2 |
| ρcalcmg/mm3 | 1.511 | 1.244 |
| m/mm‑1 | 0.12 | 0.097 |
| F(000) | 736 | 314 |
| Crystal size/mm3 | 0.28 × 0.05 × 0.01 | 0.33 × 0.14 × 0.11 |
| 2Θ range for data collection | 4.14 to 50° | 3.24 to 61.08° |
| Index ranges | −5 ≤ h ≤ 9, −18 ≤ k ≤ 18, −15 ≤ l ≤ 14 | −8 ≤ h ≤ 6, −14 ≤ k ≤ 10, −17 ≤ l ≤ 17 |
| Reflections collected | 7,052 | 2,552 |
| Independent reflections | 2,704[R(int) = 0.0721] | 2,237[R(int) = 0.0358] |
| Data/restraints/parameters | 2,704/3/241 | 2,237/12/254 |
| Goodness-of-fit on F2 | 0.986 | 0.804 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0532, wR2 = 0.1121 | R1 = 0.0462, wR2 = 0.1239 |
| Final R indexes [all data] | R1 = 0.1117, wR2 = 0.1358 | R1 = 0.0555, wR2 = 0.1354 |
| Largest diff. peak/hole/e Å−3 | 0.305/−0.264 | 0.43/−0.34 |
2.3. Anti-AChE Assay
| Compounds | Molecular weight | AChE Inhibition (%)(Final conc. = 1 × 10−4 M) | DPPH(IC50, μg/mL) | FRAP value(Mean ± SD) |
|---|---|---|---|---|
| 1 | 184.15 | 38.0 ± 1.3 | 1.210 ± 0.002 | 81,633.30 ± 0.075 |
| 2 | 381.18 | 77.0 ± 1.8 | 1.140 ± 0.001 | 62,200.00 ± 0.083 |
| 3 | 336.73 | 68.9 ± 1.8 | 1.400 ± 0.002 | 35,740.00 ± 0.011 |
| 4 | 332.31 | 48.5 ± 2.5 | 1.220 ± 0.001 | 30,080.00 ± 0.054 |
| 5 | 287.27 | 16.4 ± 1.4 | 1.460 ± 0.001 | 22,946.70 ± 0.004 |
| 6 | 329.31 | 71.5 ± 1.7 | 2.300 ± 0.001 | 23,340.00 ± 0.021 |
| Propidium | - | 54.5 ± 1.6 | - | - |
| Tacrine | - | 51.2 ± 1.6 | - | - |
| Ascorbic acid | - | - | 2.260 ± 0.001 | 19,400.00 ± 0.007 |
| BHT | - | - | - | 187.3 ± 2.6 |
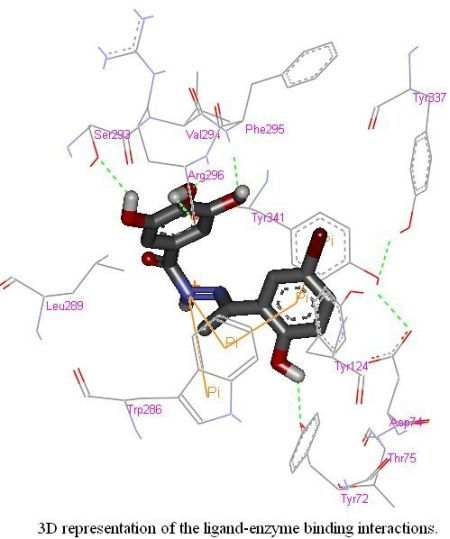
2.4. Molecular Docking


2.5. Antioxidant Assays

3. Experimental Section
3.1. General
3.2. Gallic Hydrazide (1)

3.3. N-(1-(5-Bromo-2-hydroxyphenyl)-ethylidene)-3,4,5-trihydroxybenzohydrazide (2)

3.4. N-(1-(5-Chloro-2-hydroxyphenyl)-ethylidene)-3,4,5-trihydroxybenzohydrazide (3)

3.5. N-(1-(2-Hydroxy-5-methoxyphenyl)-ethylidene)-3,4,5-trihydroxybenzohydrazide (4)

3.6. 3,4,5-Trihydroxybenzoic Acid [1-Pyridylethylidene] Hydrazide (5)

3.7. N'-(1-(6-Acetylpyridin-2-yl)ethylidene)-3,4,5-trihydroxybenzohydrazide (6)

3.8. X-ray Crystallography
3.9. Anti-AChE Assay
3.10. Molecular Modeling Evaluations
3.11. Antioxidant Activity
3.11.1. DPPH (1,1-Diphenyl-2-picrylhydrazyl) Assay
3.11.2. FRAP Assay
3.12. Statistical Analysis
4. Conclusions
Supplementary Data
Acknowledgements
References and Notes
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fatima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Creaven, B.S.; Duff, B.; Egan, D.A.; Kavanagh, K.; Rosair, G.; Thangella, V.R. Anticancer and antifungal activity of copper(II) complexes of quinolin-2(1H)-one-derived Schiff bases. Inorg. Chim. Acta 2010, 363, 4048–4058. [Google Scholar] [CrossRef]
- Ceyhan, G.; Çelik, C.; Uruş, S.; Demirtaş, İ.; Elmastaş, M.; Tümer, M. Antioxidant, electrochemical, thermal, antimicrobial and alkane oxidation properties of tridentate Schiff base ligands and their metal complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 81, 184–198. [Google Scholar] [CrossRef]
- Qiao, X.; Ma, Z.-Y.; Xie, C.-Z.; Xue, F.; Zhang, Y.-W.; Xu, J.-Y. Study on potential antitumor mechanism of a novel Schiff Base copper(II) complex: Synthesis, crystal structure, DNA binding, cytotoxicity and apoptosis induction activiy. J. Inorg. Biochem. 2011, 105, 728–737. [Google Scholar] [CrossRef]
- Xu, D.; Ma, S.; Du, G.; He, Q.; Sun, D. Synthesis, characterization, and anticancer properties of rare earth complexes with Schiff base and o-phenanthroline. J. Rare Earth. 2008, 26, 643–647. [Google Scholar] [CrossRef]
- Rice-Evans, C. Implications of the mechanisms of action of tea polyphenols as antioxidants in vitro for chemoprevention in humans. In Proceedings of the Society for Experimental Biology and Medicine, London, UK, 1999; Volume 220, pp. 262–266.
- Clemetso, C.A.B.; Andersen, L. Plant Polyphenols as Antioxidants for Ascorbic Acid; Clemetso, C.A.B., Andersen, L., Eds.; New York Academy of Sciences: New York, NY, USA, 1966; Volume 136, pp. 341–376. [Google Scholar]
- Sun-Waterhouse, D.; Chen, J.; Chuah, C.; Wibisono, R.; Melton, L.D.; Laing, W. Kiwifruit-based polyphenols and related antioxidants for functional foods: Kiwifruit extract-enhanced gluten-free bread. Int. J. Food Sci. Nutr. 2009, 60, 251–264. [Google Scholar] [CrossRef]
- Kuhn, D.J.; Lam, W.H.; Kazi, A.; Daniel, K.G.; Song, S.J.; Chow, L.M.C. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front Biosci. 2005, 10, 1010–1023. [Google Scholar] [CrossRef]
- Samoylenko, O.; Zaletok, S.; Orlovsky, O.; Gogol, S.; Klenov, O.; Shapochka, D. Additive antitumor effect of plant polyphenols and a synthetic inhibitors of polyamines biosynthesis. Breast 2011, 20, S22–S23. [Google Scholar]
- Claudine, M.; Andrzej, M.; Augustin, S. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- Capasso, R.; Evidente, A.; Tremblay, E.; Sala, A.; Santoro, C.; Cristinzio, G. Direct and mediated effects on Bactrocera oleae (Gmelin) (Diptera, Tephritidae) of natural polyphenols and some of related synthetic compounds: Structure-activity relationships. J. Chem. Ecol. 1994, 20, 1189–1199. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Mayeux, R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004, 3, 579–587. [Google Scholar] [CrossRef]
- Dai, Q.; Borenstein, A.R.; Wu, Y.; Jackson, J.C.; Larson, E.B. Fruit and vegetable juices and Alzheimer’s disease: The Kame Project. Am. J. Med. 2006, 119, 751–759. [Google Scholar] [CrossRef]
- Bourn, D.; Prescott, J. A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. Crit. Rev. Food Sci. Nutr. 2002, 42, 1–34. [Google Scholar]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317–325. [Google Scholar]
- El-Ansary, A.L.; Abdel-Fattah, H.M.; Abdel-Kader, N.S. Synthesis, spectral, thermal and magnetic studies of Mn(II), Ni(II) and Cu(II) complexes with some benzopyran-4-one Schiff bases. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 522–528. [Google Scholar] [CrossRef]
- Khan, T.A.; Naseem, S.; Khan, S.N.; Khan, A.U.; Shakir, M. Synthesis and spectral characterization of 14- and 16-membered tetraazamacrocyclic Schiff base ligands and their transition metal complexes and a comparative study of interaction of calf thymus DNA with copper(II) complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 622–629. [Google Scholar] [CrossRef]
- Nath, M.; Saini, P.K.; Kumar, A. New di- and triorganotin(IV) complexes of tripodal Schiff base ligand containing three imidazole arms: Synthesis, structural characterization, anti-inflammatory activity and thermal studies. J. Organomet. Chem. 2010, 695, 1353–1362. [Google Scholar] [CrossRef]
- Issa, R.M.; Khedr, A.M.; Rizk, H.F. UV-vis, IR and 1H NMR spectroscopic studies of some Schiff bases derivatives of 4-aminoantipyrine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 621–629. [Google Scholar] [CrossRef]
- Pang, S.; Liang, Y. Studies on charge transfer properties from mixture of Schiff base and zinc complex in Langmuir-Blodgett film by UV-vis absorption and Fourier transform infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 435–439. [Google Scholar] [CrossRef]
- Suleiman Gwaram, N.; Khaledi, H.; Mohd Ali, H.; Robinson, W.T.; Abdulla, M.A. N'-[1-(5-Bromo-2-hydroxyphenyl)ethylidene]-3,4,5-trihydroxybenzohydrazide dimethyl sulfoxide solvate trihydrate. Acta Crystallogr. 2010, E66, o721. [Google Scholar]
- Heider, E.M.; Harper, J.K.; Grant, D.M.; Hoffman, A.; Dugan, F.; Tomere, D.P.; O’Neille, K.L. Unusual antioxidant activity in a benzoic acid derivative: A proposed mechanism for citrinin. Tetrahedron 2006, 62, 1199–1208. [Google Scholar]
- Kadoma, Y.; Atsumi, T.; Okada, N.; Ishihara, M.; Yokoe, I.; Fujisawa, S. Radical-scavenging activity of the reaction products of isoeugenol with thiol, thiophenol, mercaptothiazoline or mercaptomethylimidazole using the induction period method. Molecules 2007, 12, 130–138. [Google Scholar] [CrossRef]
- Kryger, G.; Harel, M.; Giles, K.; Toker, L.; Velan, B.; Lazar, A.; Kronman, C.; Barak, D.; Ariel, N.; Shafferman, A. Structures of recombinant native and e202q mutant human acetylcholinesterase complexed with the snake-venom toxin fasciculin-ii. Acta Crystallogr. D Biol. Crystallogr. 2000, 56, 1385–1394. [Google Scholar] [CrossRef]
- Wiesner, J.; Kriz, Z.; Kuca, K.; Jun, D.; Koca, J. Acetylcholinesterases—The structural similarities and differences. J. Enzyme Inhib. Med. Chem. 2007, 22, 417–424. [Google Scholar] [CrossRef]
- Heo, B.G.; Park, Y.S.; Chon, S.U.; Lee, S.Y.; Cho, J.Y.; Gorinstein, S. Antioxidant activity and cytotoxicity of methanol extracts from aerial parts of Korean salad plants. Biofactors 2007, 30, 79–89. [Google Scholar] [CrossRef]
- Khaledi, H.; Alhadi, A.A.; Yehye, W.A.; Ali, H.M.; Abdulla, M.A.; Hassandarvish, P. Antioxidant, cytotoxic activities, and structure-activity relationship of gallic acid-based indole derivatives. Arch. Pharm. 2011, 344, 703–709. [Google Scholar] [CrossRef]
- Stockdale, M.; Selwyn, M.J. Effects of ring substituents on the activity of phenols as inhibitors and uncouplers of mitochondrial respiration. Eur. J. Biochem. 1971, 21, 565–574. [Google Scholar]
- Bruker APEX2 and SAINT. Bruker AXS Inc: Madison, WI, USA, 2007.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112. [Google Scholar] [CrossRef]
- Barbour, L.J. X-Seed—A software tool for supramolecular crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Inhibition of acetylcholinesterase activity as effect criterion in acute test with juvenile Daphnia magna. Chemosphere 1996, 32, 721–738. [Google Scholar]
- Laskwoski, R.A. PDBsum: Summaries and analyses of PDB structure. Nucleic Acids Res. 2001, 29, 221–222. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Wallace, C.A.; Laskowski, A.R.; Thornton, M.J. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Choi, W.C.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 1999, 299, 15–27. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gwaram, N.S.; Ali, H.M.; Abdulla, M.A.; Buckle, M.J.C.; Sukumaran, S.D.; Chung, L.Y.; Othman, R.; Alhadi, A.A.; Yehye, W.A.; Hadi, A.H.A.; et al. Synthesis, Characterization, X-ray Crystallography, Acetyl Cholinesterase Inhibition and Antioxidant Activities of Some Novel Ketone Derivatives of Gallic Hydrazide-Derived Schiff Bases. Molecules 2012, 17, 2408-2427. https://doi.org/10.3390/molecules17032408
Gwaram NS, Ali HM, Abdulla MA, Buckle MJC, Sukumaran SD, Chung LY, Othman R, Alhadi AA, Yehye WA, Hadi AHA, et al. Synthesis, Characterization, X-ray Crystallography, Acetyl Cholinesterase Inhibition and Antioxidant Activities of Some Novel Ketone Derivatives of Gallic Hydrazide-Derived Schiff Bases. Molecules. 2012; 17(3):2408-2427. https://doi.org/10.3390/molecules17032408
Chicago/Turabian StyleGwaram, Nura Suleiman, Hapipah Mohd Ali, Mahmood Ameen Abdulla, Michael J. C. Buckle, Sri Devi Sukumaran, Lip Yong Chung, Rozana Othman, Abeer A. Alhadi, Wageeh A. Yehye, A. Hamid A. Hadi, and et al. 2012. "Synthesis, Characterization, X-ray Crystallography, Acetyl Cholinesterase Inhibition and Antioxidant Activities of Some Novel Ketone Derivatives of Gallic Hydrazide-Derived Schiff Bases" Molecules 17, no. 3: 2408-2427. https://doi.org/10.3390/molecules17032408