Spectral Properties of Substituted Coumarins in Solution and Polymer Matrices
Abstract
:1. Introduction

2. Results and Discussion
2.1. Absorption Spectra

| Comp./Medium | 1 λA / log ε(nm) | 2 λA / log ε(nm) | 5 λA / log ε(nm) | 6 λA / log ε(nm) | 7 λA / log ε(nm) |
|---|---|---|---|---|---|
| MeOH | 278 / 3.78 * | 287 / 4.37 * | |||
| MeOH | 310 / 3.56 * | 315 / 4.32 * | |||
| MeOH | 354 / 3.10 * | 359 / 4.30 | 360 / 4.03 * | 361 / 4.41 | 361 / 3.96 * |
| CHCl3 | 307 / 4.30 | 321 / 4.27 | |||
| CHCl3 | 348 / 4.12 | 360 / 4.30 | 356 / 4.24 | 364 / 4.37 | 370 / 4.39 |
| PMMA | 306 / 3.90 | 312 / 4.31 | |||
| PMMA | 351 / 3.72 | 361 / 4.15 | 347 / 4.23 | 364 / 4.15 | 368 / 4.20 |
| PVC | 311 / 3.85 | 321 / 4.12 | |||
| PVC | 357 / 3.67 | 364 / 4.24 | 357 / 4.08 | 368 / 4.26 | 372 / 4.32 |
| PS | 314 / 3.85 | 323 / 4.06 | |||
| PS | 351 / 3.74 | 365 / 4.11 | 357 / 4.07 | 365 / 4.21 | 372 / 4.22 |
| Comp./Medium | λA / log ε(nm) | Δν1/2(cm−1) | λF(nm) | νA–νF(cm−1) | ΦF | τ(ns) | kF(109 s−1) | knr(109 s−1) |
|---|---|---|---|---|---|---|---|---|
| 3 | ||||||||
| MeOH | 436 / 4.49 | 3355 | 492 | 2611 | 0.40 ± 0.06 | 1.1 ± 0.1 | 0.37 | 0.54 |
| CHCl3 | 441 / 4.66 | 2580 | 470 | 1399 | 0.44 ± 0.07 | 2.6 ± 0.1 | 0.17 | 0.22 |
| PMMA | 440 / 4.39 | 2939 | 475 | 1675 | 0.81 ± 0.12 | 3.3 ± 0.2 | 0.24 | 0.06 |
| PVC | 446 / 4.56 | 2918 | 482 | 1675 | 1.08 ± 0.16 | 4.0 ± 0.2 | 0.27 | - |
| PS | 438 / 4.41 | 2555 | 468 | 1464 | 0.22 ± 0.03 | 2.5 ± 0.1 | 0.09 | 0.31 |
| 4 | ||||||||
| MeOH | 431 / 4.48 | 3402 | 503 | 3321 | 0.64 ± 0.10 | 1.8 ± 0.1 | 0.36 | 0.20 |
| CHCl3 | 433 / 4.61 | 3811 | 492 | 2769 | 0.49 ± 0.07 | 1.8 ± 0.1 | 0.27 | 0.28 |
| PMMA | 432 / 4.35 | 3894 | 504 | 3307 | 1.08 ± 0.16 | 3.6 ± 0.2 | 0.30 | - |
| PVC | 440 / 4.43 | 3805 | 505 | 2925 | 1.10 ± 0.17 | 2.3 ± 0.1 | 0.48 | - |
| PS | 429 / 4.42 | 4437 | 505 | 3508 | 0.25 ± 0.04 | 2.6 ± 0.1 | 0.10 | 0.29 |
| 8 | ||||||||
| MeOH | 373 / 4.54 | 5431 | 474 | 5713 | 0.007 ± 0.001 | |||
| CHCl3 | 375 / 4.37 | 5343 | 464 | 4973 | 0.011 ± 0.002 | 3.3 ± 0.2 | 0.003 | 0.30 |
| PMMA | 375 / 4.25 | 4842 | 463 | 5068 | 0.30 ± 0.05 | 2.3 ± 0.1 | 0.13 | 0.30 |
| PVC | 379 / 4.38 | 4547 | 466 | 4926 | 0.29 ± 0.04 | 1.9 ± 0.1 | 0.15 | 0.37 |
| PS | 375 / 4.14 | 4876 | 465 | 5161 | 0.06 ± 0.01 | 0.7 ± 0.04 | 0.09 | 1.34 |
| Comp./Medium | ES1(kJ mol−1) | f | τ0(ns) | τF-calc(ns) | kF-calc(109 s−1) | knr-calc(109 s−1) |
|---|---|---|---|---|---|---|
| 3 | ||||||
| MeOH | 258 | 0.33 | 8.5 | 3.4 | 0.12 | 0.18 |
| CHCl3 | 263 | 0.34 | 8.6 | 3.8 | 0.12 | 0.15 |
| PMMA | 261 | 0.31 | 9.3 | 7.5 | 0.11 | 0.03 |
| PVC | 258 | 0.46 | 6.5 | 7.0 | 0.15 | - |
| PS | 264 | 0.29 | 1.0 | 0.2 | 0.99 | 3.57 |
| 4 | ||||||
| MeOH | 256 | 0.34 | 8.3 | 5.3 | 0.12 | 0.07 |
| CHCl3 | 259 | 0.45 | 6.2 | 3.0 | 0.16 | 0.17 |
| PMMA | 267 | 0.38 | 7.4 | 8.0 | 0.14 | - |
| PVC | 253 | 0.44 | 6.6 | 7.3 | 0.15 | - |
| PS | 265 | 0.51 | 5.5 | 1.4 | 0.18 | 0.54 |
| 8 | ||||||
| MeOH | 282 | 0.61 | 3.4 | 0.02 | 0.29 | 45.0 |
| CHCl3 | 284 | 0.36 | 5.9 | 0.06 | 0.17 | 15.0 |
| PMMA | 285 | 0.25 | 8.5 | 2.51 | 0.12 | 0.3 |
| PVC | 283 | 0.47 | 4.6 | 1.32 | 0.22 | 0.5 |
| PS | 285 | 0.29 | 7.3 | 0.43 | 0.14 | 2.2 |

2.2. Fluorescence Spectra

3. Experimental
3.1. General
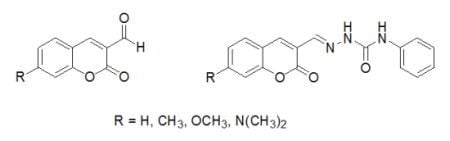
3.2. Synthesis of Coumarin Derivatives

3.3. Reaction of Aldehydes with Phenylsemicarbazide
3.4. Spectral Measurements
3.5. Relationships in the Calculation of Spectral Properties

4. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; Volume Chapter 3, p. 280. [Google Scholar]
- Valeur, B. Molecular Fluorescence: Principles and Aplications; Wiley-Verlag Chemie GmbH.: Weinheim, Germany, 2001; Volume Chapter 3, p. 79. [Google Scholar]
- Prasanna de Silva, A.; Gunaratante, H.Q.N.; Gunnlaugsson, Th.; Huxley, A.J.M.; Mc Coy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar]
- Prasanna de Silva, A.; Vance, T.P.; West, M.E.S.; Wright, G.D. Bright molecules with sense, logic, numeracy and utility. Org. Biomol. Chem. 2008, 6, 2468–2481. [Google Scholar] [CrossRef]
- Acceta, R.; Corradini, R.; Marcelli, R. Enantioselective sensing by luminescence. Top. Curr. Chem. 2011, 300, 175–216. [Google Scholar]
- Jones, G., II; Jackson, W.R.; Choi, C.Y.; Bergman, W.R. Solvent effect on emission yield and lifetime for coumarin laser dyes. Requirement for rotatory decay mechanism. J. Phys. Chem. 1985, 89, 294–300. [Google Scholar]
- Horng, M.L.; Gardeski, J.A.; Papazyan, A.; Maroncelli, M. Subpicosecond measurement of polar salvation dynamics: Coumarin 153 revisited. J. Phys. Chem. 1995, 99, 17311–17337. [Google Scholar] [CrossRef]
- Arbeloa, T.L.; Arbeloa, F.L.; Arbeloa, I.L. Influence of fluorinated group on the photophysics of 7-aminocoumarins. J. Lumin. 1996, 68, 149–155. [Google Scholar] [CrossRef]
- Du, D.M.; Wang, Y.M.; Meng, J.B. Micro-environmental effects on the photochemical and photophysical processes of long chain coumarin esters. Chem. J. Chin. Univ.-Chin. 1998, 19, 1611–1613. [Google Scholar]
- Raju, B.B.; Costa, S.M.B. Photophysical properties of 7-diethylaminocoumarin dyes in dioxane-water mixtures: Hydrogen bonding, dielectric enrichment and polarity effects. Phys. Chem. Chem. Phys. 1999, 1, 3539–3547. [Google Scholar]
- Ramalingam, A.; Sivaram, B.M.; Palamisamy, P.K.; Masilamani, V. Photophysics of TICT states of 7-diethylamino-4-methyl coumarin dye by energy transfer techniques. Spectrochim. Acta Part A-Mol. Biomolec. Spect. 2000, 56, 1205–1210. [Google Scholar] [CrossRef]
- Morimoto, A.; Yatsuhashi, T.; Shimada, T.; Biczok, L.; Tryk, D.A.; Inoue, H. Radiationless deactivation of an intramolecular charge transfer excited state through hydrogen bonding: Effect of molecular structure and hard-soft anionic character in the excited state. J. Phys. Chem. A 2001, 105, 10488–10496. [Google Scholar] [CrossRef]
- Morlet-Savary, F.; Ley, C.; Jacques, P.; Fouasier, J.P. Photophysics of a bridged 7-diethylamino-4-methylcoumarin C102: Studying the hydrogen bonding effect by time resolved stimulated emission. J.Phys. Chem. A 2001, 105, 11026–11033. [Google Scholar]
- Satpati, A.K.; Senthilkumar, S.; Kumbhakar, M.; Nath, S.; Maity, D.K.; Pal, H. Investigations of the solvent polarity effect on the photophysical properties of coumarin-7 dye. Photochem. Photobiol. 2005, 81, 270–278. [Google Scholar] [CrossRef]
- Satpati, A.K.; Kumbhakar, M.; Maity, D.K.; Pal, H. Photophysical investigations of the solvent polarity effect on properties of coumarin-6 dye. Chem. Phys. Lett. 2005, 407, 114–118. [Google Scholar] [CrossRef]
- Mandal, P.K.; Paul, A.; Samanta, A. Fluorescence studies of environmentally benign solvents: Solvation dynamics of coumarin 102 in [BMIM][BF4]. Res. Chem. Intermed. 2005, 31, 575–583. [Google Scholar] [CrossRef]
- Raikar, U.S.; Renuka, C.G.; Nadaf, Y.F.; Mulimani, B.G.; Karguppikar, A.M. Rotational diffusion and solvatochromic correlation of coumarin 6 laser dye. J. Fluoresc. 2006, 16, 847–854. [Google Scholar] [CrossRef]
- Raikar, U.S.; Renuka, C.G.; Nadaf, Y.F.; Mulimani, B.G.; Karguppikar, A.M.; Soudagar, M.K. Solvent effects on the absorption and fluorescence spectra of coumarins 6 and 7 molecules: Determination of ground state and excited state dipole moment. Spectrochim. Acta A 2006, 65, 673–677. [Google Scholar] [CrossRef]
- Tablet, C.; Hildebrand, M. Quenching of the fluorescence of carboxy-5,6-benzocoumarin by aromatic amines. J. Photochem. Photobiol. A Chem. 2007, 189, 73–79. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Y.; Shi, M.; Wu, F. The synthesis of novel coumarin dyes and the study of their photoreaction properties. Dyes Pigments 2007, 75, 104–110. [Google Scholar] [CrossRef]
- Ammar, H.; Abid, S.; Fery-Forgues, S. Synthesis and spectroscopic study of new biscoumarin dyes based on 7-(4-methylcoumarinyl) diesters. Dyes Pigments 2008, 78, 1–7. [Google Scholar] [CrossRef]
- Winnik, F.M.; Regismond, S.T.A. Fluorescence methods in the study of the interactions of surfactants with polymers. Colloids Surf. A Physicochem. Eng. Asp. 1996, 118, 1–36. [Google Scholar] [CrossRef]
- De Paula, R.; De Hora, A.E.; Machado De Miranda, J.A. 3-Benzoxazol-2-yl-7-(N,N-diethylamino)/chromen-2-one as a fluorescence probe for investigation of micellar microenvironments. J. Photochem. Photobiol. A Chem. 2004, 165, 109–114. [Google Scholar] [CrossRef]
- Das, K.; Jain, B.; Gupta, P.K. Photophysics of coumarin 500 and coumarin 151 in AOT revers micelles. Chem. Phys. Lett. 2005, 410, 160–164. [Google Scholar] [CrossRef]
- Tamoto, Y.; Segawa, H.; Shirota, H. Solvation dynamics in aqueous anionic and cationic micelle solutions: Sodium alkyl sulfate and alkyltrimethylamonium bromide. Langmuir 2005, 21, 3757–3764. [Google Scholar] [CrossRef]
- Scypinski, S.; Drake, J.M. Photophysics of coumarin inclusion complexes with inverted comples formation. J. Phys. Chem. 1985, 89, 2432–2435. [Google Scholar] [CrossRef]
- Chakraborty, A.; Seth, D.; Chakrabarty, D.; Sarkar, N. Photoinduced intermolecular electron transfer from aniline to 7-amino coumarin dyes in the surface of β-cyclodextrin. Spectrochim. Acta Part A 2006, 64, 801–808. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Anpo, M.; Xue, M.; Liu, Y. Photophysical properties of coumarin-6 molecules incorporated within MCM-48. Materials Lett. 2005, 59, 2120–2123. [Google Scholar]
- Guan, H.; Zhu, L.; Zhou, H.; Tang, H. Rapid probing of photoactivity on titania based self-cleaning materials using 7-hydroxycoumarin fluorescent probe. Anal. Chim. Acta 2008, 608, 73–78. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shimada, R.; Maeda, A.; Kojima, K.; Uchida, K. Photophysics of coumarin 4 doped-amorphous silica glasses prepared by sol-gel method. J. Lumin. 1996, 68, 187–192. [Google Scholar] [CrossRef]
- Stathatos, E.; Lianos, P.; Lavrencic-Stangar, U.; Orel, B. Study of laser action of coumarine-153 incorporated in sol-gel made silica/poly(propylene oxide) nanocomposite gels. Chem. Phys. Lett. 2001, 345, 381–385. [Google Scholar] [CrossRef]
- Takahashi, Y.; Maeda, A.; Kojima, K. Optical pH sensing characteristics in dye-doped sol-gel coating film base on the energy transfer. Jpn. J. Appl. Phys. 1 2003, 42, 4369–4377. [Google Scholar]
- Yamaguchi, A.; Amino, Y.; Shima, K.; Suzuki, S.; Yamashita, T.; Teramae, N. Local environments of coumarin dyes within mesostructured silica-surfactant nanocomposites. J. Phys. Chem. B 2006, 110, 3910–3916. [Google Scholar]
- Sabatini, C.A.; Pereira, R.V.; Gehlen, M.H. Fluorescence modulation of acridin and coumarine dyes by silver nanoparticles. J. Fluoresc. 2007, 17, 377–382. [Google Scholar] [CrossRef]
- Soutar, I. The application of Luminescence Technique in Polymer Science. Polym. Int. 1991, 26, 35–49. [Google Scholar] [CrossRef]
- Morawetz, H. On the versatility of fluorescence technique in polymer research. J. Polym. Sci. A Polym. Chem. 1999, 37, 1725–1735. [Google Scholar] [CrossRef]
- Sarker, A.M.; Kaneko, Y.; Neckers, D.C. Photochemistry and photophysics of novel photoinitiators: N,N,N-Tributyl-N-(4-methylene-7-methoxycoumarin)ammonium borates. J. Photochem. Photobiol. 1998, 117, 67–74. [Google Scholar] [CrossRef]
- Oh, J.K.; Wu, J.; Winnik, M.A.; Croun, G.P.; Rademacher, J.; Farwaha, R. Emulsion copolymerization of vinyl acetate and butylacetate in the presence of fluorescent dyes. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1594–1604. [Google Scholar] [CrossRef]
- Kaholek, M.; Hrdlovič, P. Spectral properties of coumarin derivatives substituted at position 3. Effect of polymer matrix. J. Photochem. Photobiol. A Chem. 1997, 108, 283–288. [Google Scholar] [CrossRef]
- Kaholek, M.; Hrdlovič, P. Characteristics of excited states of 3-substituted coumarin derivatives and transfer of electronic energy to N-oxyl radicals. J. Photochem. Photobiol. A Chem. 1999, 127, 45–55. [Google Scholar] [CrossRef]
- Kaholek, M.; Hrdlovič, P.; Bartos, J. Singlet probes based on coumarin derivatives substituted at position 3;spectral properties in solution and polymer matrices. Polymer 2000, 41, 991–1001. [Google Scholar]
- Jones, G.; Jimenez, J.A.C. Azole-linked coumarin dyes as fluorescence probes of domain-forming polymers. J. Photochem. Photbiol. BBiol. 2001, 65, 5–12. [Google Scholar] [CrossRef]
- Kim, C.; Trajkovskaja, A.; Wallace, J.U.; Chen, S.H. New insight into photoalignment of liquid crystals on coumarin containing polymer films. Macromolecules 2006, 39, 3817–3823. [Google Scholar] [CrossRef]
- Ley, J.G.E.; Tirumalasetty, P.P. Release chracteristics of polymethylmethcrytate nanospheres containing coumarin 6. J. Microencapsul. 2003, 20, 653–659. [Google Scholar]
- Mansour, A.F.; Killa, H.M.A.; El-Wanees, S.A.; El-Sayed, M.Y. Laser dyes doped with with (ST-co-MMA) as fluorescent solar collectors and their field performance. Polym. Testing 2005, 24, 519–525. [Google Scholar] [CrossRef]
- Felorzabihi, N.; Haley, J.C.; Bardajee, G.; Winnik, M.A. Systematic study of the fluorescence decay of amino-coumarins dyes in polymer matrices. J. Polym. Sci. B Polym. Phys. 2007, 45, 2333–2343. [Google Scholar]
- Fu, Q.; Cheng, L.; Zhang, Y.; Shi, W. Preparation and reversible photocrosslinking/photo-cleavage of 4-methylcoumarin functionalized hyperbranched polyester. Polymer 2008, 49, 4981–4988. [Google Scholar] [CrossRef]
- Bayer, M.; Steger, D.; Fischer, K. The Luminescence of lignign-containing pulps—A comparison with fluorescence of model compounds in several media. J. Photochem. Photobiol. A Chem. 1993, 76, 217–224. [Google Scholar] [CrossRef]
- Fischer, K.; Spange, S.; Fischer, S.; Bellmann, C.; Adams, J. Probing the surface polarity of native cellulose using genuine solvatochromic dyes. Cellulose 2002, 9, 31–40. [Google Scholar] [CrossRef]
- Nad, S.; Pal, H. Electron transfer from diphenyl and triphenyl amines to excited coumarin dyes. J. Photochem. Photobiol. A Chem. 2000, 134, 9–15. [Google Scholar] [CrossRef]
- Nad, S.; Pal, H. Electron transfer from aromatic amines to excited coumarin dyes. Fluorescence qenching and picosecond transient absorption studies. J. Phys. Chem. 2000, 104, 673–680. [Google Scholar]
- Coenjarts, C.; Garcia, O.; Llauger, L.; Vinette, A.L.; Scaiano, J.C. Mapping photogenerated radicals in thin polymer films: Fluorescence imaging using a prefluorescent radical probe. J. Am. Chem. Soc. 2003, 125, 620–621. [Google Scholar]
- Scaiano, J.C.; Aliaga, C.; Chretien, M.N.; Frenette, M.; Focsaneanu, K.S.; Mikelson, L. Fluorescence sensor application as detector for DNA damage, free radical formation, and in microlithography. Pure Appl. Chem. 2005, 77, 1009–1018. [Google Scholar]
- Danko, M.; Szabo, E.; Hrdlovič, P. Synthesis and spectral characterization of fluorescence dyes based on coumarin fluorophore and hindered amine stabilizer in solution and polymer matrices. Dyes Pigments 2011, 90, 129–138. [Google Scholar] [CrossRef]
- Viriota, M.L.; Carre, M.C.; Geoffroy-Chapotot, C.; Brembilla, A.; Muller, S.; Stoltz, J.-F. Molecular rotors as fluorescent probes for biological studies. Clin. Hemorheol. Microcirc. 1998, 19, 151–160. [Google Scholar]
- Keskin, S.S.; Aslan, N.; Bayrakceken, F. Optical properties and chemical behavior of laser dye coumarin-500and the influence of atmospheric corona discharge. Spectrochem. Acta A Biomol. Spectros. 2009, 72, 254–259. [Google Scholar] [CrossRef]
- Christie, R.M.; Morgan, K.M.; Islam, M.S. Molecular design and synthesis of arylsulfonated coumarin fluorescent dyes and their application to textile. Dyes Pigments 2008, 76, 741–747. [Google Scholar] [CrossRef]
- Leray, I.; Habib-Jiwan, J.-L.; Branger, C.; Soumillion, J.-Ph.; Valeur, B. Ion responsive fluorescent compounds VI. Ccoumarin 153 linked to rigid crowns for improvement of selectivity. J. Photochem. Photobiol. A Chem. 2000, 135, 163–169. [Google Scholar] [CrossRef]
- Nakazono, M.; Saita, K.; Kurihara, Ch.; Nanbu, S.; Zaitsu, K. Intramolecular energy transfer in š-amino-N-(7-methoxy-4′-methylcoumaryl)phthalimide. J. Photochem.Photobiol. A Chem. 2009, 208, 21–26. [Google Scholar] [CrossRef]
- Flašík, R.; Stankovičová, H.; Gáplovský, A.; Donovalová, J. Synthesis and study of novel derivatives potentially utilizable as memory media. Molecules 2009, 14, 4838–4848. [Google Scholar]
- Hrdlovič, P.; Donovalová, J.; Stankovičová, H.; Gaplovský, A. Influence of polarity of solvents on spectral properties of Bichromophoric Coumarins. Molecules 2010, 15, 8915–8932. [Google Scholar]
- Bangar, R.B.; Varandajaran, T.S. Substituent and solvent effects on the twisted intramolecular charge transfer of three new 7-(diethylamino)coumarin-3-aldehyde derivatives. J. Phys. Chem. 1994, 98, 8903–8905. [Google Scholar] [CrossRef]
- Preat, J.; Jacquemin, D.; Wathelet, V.; André, J.-M.; Perpète, E.A. TD-DFT Investigation of the UV Spectra of Pyranone Derivatives. J. Phys. Chem. A 2006, 110, 8144–8150. [Google Scholar]
- René, L.; Lefebvre, A.; Auzou, G. An Easy Conversion of 2H-chromenes into Coumarins. An Entry to 3-Formyl Coumarins. Synthesis 1986, 7, 567–569. [Google Scholar]
- Auzov, G.; René, L. Obtention de dialkylamino-2 cyano-3 2H-chromènes. J. Heterocycl. Chem. 1986, 23, 955–956. [Google Scholar] [CrossRef]
- Birks, J.B. Photophysics of Aromatic Molecules; Willey-Interscience: New York, NY, USA, 1968; Volume Chapter 4, pp. 121–127. [Google Scholar]
- Kawski, A.; Kubicki, A.; Kuklinski, B.; Gryczynski, I. Unusual absorption and fluorescence properties of 1,6-diphenyl-1,3,5-hexatriene in poly(vinyl alcohol) film. J. Photochem. Photobiol. A: Chem. 1993, 71, 161. [Google Scholar] [CrossRef]
- Demas, J.N.; Adamson, A.W. Evaluation of photoluminescence lifetimes. J. Phys. Chem. 1971, 57, 2463. [Google Scholar]
- Demas, J.N. Excited State Lifetime Measurements; Academic Press: New York, NY, USA, 1973; Volume Appendix 4, p. 245. [Google Scholar]
- Sample Availability: Samples of compounds 1–8 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Donovalová, J.; Cigáň, M.; Stankovičová, H.; Gašpar, J.; Danko, M.; Gáplovský, A.; Hrdlovič, P. Spectral Properties of Substituted Coumarins in Solution and Polymer Matrices. Molecules 2012, 17, 3259-3276. https://doi.org/10.3390/molecules17033259
Donovalová J, Cigáň M, Stankovičová H, Gašpar J, Danko M, Gáplovský A, Hrdlovič P. Spectral Properties of Substituted Coumarins in Solution and Polymer Matrices. Molecules. 2012; 17(3):3259-3276. https://doi.org/10.3390/molecules17033259
Chicago/Turabian StyleDonovalová, Jana, Marek Cigáň, Henrieta Stankovičová, Jan Gašpar, Martin Danko, Anton Gáplovský, and Pavol Hrdlovič. 2012. "Spectral Properties of Substituted Coumarins in Solution and Polymer Matrices" Molecules 17, no. 3: 3259-3276. https://doi.org/10.3390/molecules17033259