A Convenient One-Pot Preparation of 2-Methyl-3-(phenylthio- methyl)quinolines from Morita-Baylis-Hillman Adducts and Their Oxidation to the Corresponding Sulfones
Abstract
:1. Introduction

2. Results and Discussion


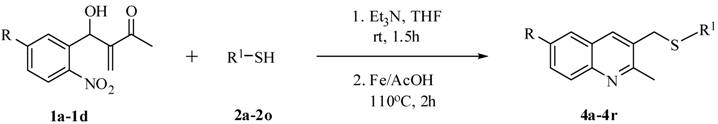 | ||||||
|---|---|---|---|---|---|---|
| Entry | MBH adduct 1 | R | R 1 | Thiol 2 | Product 4 | Yield(%) a,b |
| 1 | 1a | H | C6H5 | 2a | 4a | 66 |
| 2 | 1a | H | 4-OMe-C6H4 | 2b | 4b | 69 |
| 3 | 1a | H | 4-Br-C6H4 | 2c | 4c | 70 |
| 4 | 1a | H | 4-Cl-C6H4 | 2d | 4d | 68 |
| 5 | 1a | H | 4-F-C6H4 | 2e | 4e | 70 |
| 6 | 1a | H | 4-Me-C6H4 | 2f | 4f | 67 |
| 7 | 1a | H | 4-Et-C6H4 | 2g | 4g | 65 |
| 8 | 1a | H | 1-Naphthyl | 2h | 4h | 72 |
| 9 | 1a | H | Propyl | 2i | 4i | 69 |
| 10 | 1a | H | Hexyl | 2j | 4j | 65 |
| 11 | 1a | H | Isopropyl | 2k | 4k | 65 |
| 12 | 1a | H | 2-Naphthyl | 2l | 4l | 75 |
| 13 | 1a | H | 3,5-Me2-C6H3 | 2m | 4m | 64 |
| 14 | 1a | H | 2-OMe-C6H5 | 2n | 4n | 65 |
| 15 | 1a | H | 4-Isopropyl-C6H4 | 2o | 4o | 65 |
| 16 | 1b | Cl | C6H5 | 2a | 4p | 72 |
| 17 | 1c | F | C6H5 | 2a | 4q | 68 |
| 18 | 1d | Br | C6H5 | 2a | 4r | 66 |

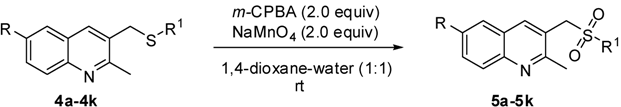 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate 4 | R | R 1 | Time (min) | Product 5 | Yield(%) a,b |
| 1 | 4a | H | C6H5 | 20 | 5a | 85 |
| 2 | 4b | H | 4-OMe-C6H4 | 20 | 5b | 89 |
| 3 | 4c | H | 4-Br-C6H4 | 40 | 5c | 81 |
| 4 | 4d | H | 4-Cl-C6H4 | 40 | 5d | 80 |
| 5 | 4e | H | 4-F-C6H4 | 35 | 5e | 82 |
| 6 | 4f | H | 4-Me-C6H4 | 20 | 5f | 87 |
| 7 | 4g | H | 4-Et-C6H4 | 20 | 5g | 86 |
| 8 | 4h | H | 1-Naphthyl | 20 | 5h | 87 |
| 9 | 4i | H | Propyl | 20 | 5i | 77 |
| 10 | 4j | H | Hexyl | 20 | 5j | 75 |
| 11 | 4k | H | Isopropyl | 30 | 5k | 78 |
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Compounds 4a–r
3.3. General Procedure for the Synthesis of Compounds 5a–k
4. Conclusions
Supplementary Materials
Acknowledgements
- Sample Availability: Samples of all compounds are available from the authors.
References and Notes
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 1997, 14, 605–608. [Google Scholar] [CrossRef]
- Bilker, O.; Lindo, V.; Panico, M.; Etiene, A.E.; Paxton, T.; Dell, A.; Rogers, M.; Sinden, R.E.; Morris, H.R. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 1998, 392, 289–292. [Google Scholar] [CrossRef]
- Edmont, D.; Rocher, R.; Plisson, C.; Chenault, J. Synthesis and evaluation of quinoline carboxyguanidines as antidiabetic agents. Bioorg. Med. Chem. Lett. 2000, 10, 1831–1834. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Fang, K.-C.; Sheu, J.-Y.; Hsu, S.-L.; Tzeng, C.-C. Synthesis and antibacterial evaluation of certain quinolone derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef]
- Zhang, X.; Shetty, A.S.; Jenekhe, S.A. Electroluminescence and photophysical properties of polyquinolines. Macromolecules 1999, 32, 7422–7429. [Google Scholar] [CrossRef]
- Xiong, H.; Kang, J.; Woods, J.M.; McCauley, J.P., Jr.; Koether, G.M.; Albert, J.S.; Hinkley, L.; Li, Y.; Gadient, R.A.; Simpson, T.R. Synthesis and SAR of sulfoxide substituted carboxyquinolines as NK3 receptor antagonists. Bioorg. Med. Chem. Lett. 2011, 21, 1896–1899. [Google Scholar]
- Bruton, G. Sulphonyl Hydroxymic Acid Derivatives as Inhibitors of S-CD23. U.S. Patent WO 2004/067502, PCT/EP2004/000954, 2004. [Google Scholar]
- Familoni, O.B.; Kaye, P.T.; Klaas, P.J. Application of Baylis-Hillman methodology in a novel synthesis of quinoline derivatives. Chem. Commun. 1998, 2563–2564. [Google Scholar]
- Familoni, O.B.; Klaas, P.J.; Lobb, K.A.; Pakade, V.E.; Kay, P.T. The Baylis-Hillman approach to quinoline derivatives. Org. Biomol. Chem. 2006, 4, 3960–3965. [Google Scholar] [CrossRef]
- Basavaiah, D.; Reddy, R.M.; Kumaragurubaran, N.; Sharada, D.S. Applications of Baylis-Hillman chemistry: One-pot convenient synthesis of functionalized (1H)-quinol-2-ones and quinolines. Tetrahedron 2002, 58, 3693–3697. [Google Scholar] [CrossRef]
- Basavaiah, D.; Rao, J.S.; Reddy, R.J. Simple, facile, and one-pot conversion of the Baylis-Hillman adducts into functionalized 1,2,3,4-tetrahydroacridines and cyclopenta[b]quinolines. J. Org. Chem. 2004, 69, 7379–7382. [Google Scholar] [CrossRef]
- Basavaiah, D.; Veeraraghavaiah, G. The Baylis-Hillman reaction: A novel concept for creativity in chemistry. Chem. Soc. Rev. 2012, 41, 68–78. [Google Scholar]
- Zhong, W.; Liu, Y.; Wang, G.; Hong, L.; Chen, Y.; Chen, X.; Zheng, Y.; Zhang, W.; Ma, W.; Shen, Y.; et al. Recent advances in construction of nitrogen-containing heterocycles from Baylis-Hillman adducts. Org. Prep. Proc. Int. 2011, 43, 1–66. [Google Scholar] [CrossRef]
- Basavaiah, D.; Reddy, B.S.; Singh, B.S. Recent contributions from the Baylis-Hillman reaction to organic chemistry. Chem. Rev. 2010, 110, 5447–5674. [Google Scholar] [CrossRef]
- Declerck, V.; Martinez, J.; Lamaty, F. aza-Baylis-Hillman reaction. Chem. Rev. 2009, 109, 1–48. [Google Scholar] [CrossRef]
- Singh, V.; Batra, S. Advances in the Baylis-Hillman reaction-assisted synthesis of cyclic frameworks. Tetrahedron 2008, 64, 4511–4574. [Google Scholar] [CrossRef]
- Madapa, S.; Tusi, Z.; Batra, S. Advances in the syntheses of quinoline and quinoline-annulated ring systems. Curr. Org. Chem. 2008, 12, 1116–1183. [Google Scholar] [CrossRef]
- Singh, V.; Hutait, S.; Batra, S. Reductive-cyclization-mediated synthesis of fused polycyclic quinolines from Baylis-Hillman adducts of acrylonitrile: Scope and limitations. Eur. J. Org. Chem. 2009, 3454–3466. [Google Scholar]
- Ciganek, E. The catalyzed α-hydroxyalkylation and α-aminoalkylation of activated olefins (the Morita-Baylis-Hillman reaction). Org. React. 1997, 51, 201–350. [Google Scholar]
- Basavaiah, D.; Rao, P.D.; Hyma, R.S. The Baylis-Hillman reaction: A novel carbon-carbon bond forming reaction. Tetrahedron 1996, 52, 8001–8062. [Google Scholar] [CrossRef]
- Basavaiah, D.; Rao, A.J.; Satyanarayana, T. Recent advances in the Baylis-Hillman reaction and applications. Chem. Rev. 2003, 103, 811–892. [Google Scholar]
- Han, E.-G.; Kim, H.J.; Lee, K.-J. Quinolines from Morita-Baylis-Hillman acetates of 2-azidobenzaldehydes. Tetrahedron 2009, 65, 9616–9625. [Google Scholar] [CrossRef]
- Cha, M.J.; Song, Y.S.; Lee, K.-J. Synthesis of symmetric diallyl disulfides from Baylis-Hillman acetates. Bull. Korean Chem. Soc. 2006, 27, 1900–1902. [Google Scholar] [CrossRef]
- Das, B.; Chowdhury, N.; Damodar, K.; Banerjee, J. A mild and efficient stereoselective synthesis of (Z)- and (E)-allyl sulfides and potent antifungal agent, (Z)-3-(4-methoxybenzylidene)thiochroman- 4-one from Morita-Baylis-Hillman acetates. Chem. Pharm. Bull. 2007, 55, 1274–1276. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, S.H.; Kim, J.N. Regioselective synthesis of poly-substituted thiophenes from Baylis-Hillman adducts. Tetrahedron Lett. 2009, 50, 6480–6483. [Google Scholar] [CrossRef]
- Ramesh, C.; Kavala, V.; Raju, B.R.; Kuo, C.W.; Yao, C.-F. Novel synthesis of indolylquinoline derivatives via the C-alkylation of Baylis-Hillman adducts. Tetrahedron Lett. 2009, 50, 4037–4041. [Google Scholar]
- Ramesh, C.; Kavala, V.; Kuo, C.W.; Raju, B.R.; Yao, C.-F. An unprecedented route for the synthesis of 3,3'-biindoles by reductive cyclization of 3-[2-nitro-1-(2-nitrophenyl)ethyl]-1H-indoles mediated by iron/acetic acid. Eur. J. Org. Chem. 2010, 3796–3801. [Google Scholar]
- Ramesh, C.; Kavala, V.; Kuo, C.W.; Yao, C.-F. Iron/acetic acid-mediated carbon degradation: A facile route for the synthesis of quinoline derivatives. Tetrahedron Lett. 2010, 51, 5234–5237. [Google Scholar] [CrossRef]
- Ramesh, C.; Raju, B.R.; Kavala, V.; Kuo, C.W.; Yao, C.-F. A simple and facile route for the synthesis of 2H-1,4-benzoxazin-3-(4H)-ones via reductive cyclization of 2-(2-nitrophenoxy)acetonitrile adducts in the presence of Fe/acetic acid. Tetrahedron 2011, 67, 1187–1192. [Google Scholar] [CrossRef]
- Reddy, D.J.; Kavala, V.; Bosco, J.J.W.; Kuo, C.W.; Yao, C.-F. An easy access to carbazolones and 2,3-disubstituted indoles. Eur. J. Org. Chem. 2011, 2360–2365. [Google Scholar]
- Shi, M.; Jiang, J.-K.; Li, C.-Q. Lewis base and L-proline co-catalyzed Baylis-Hillman reaction of arylaldehydes with methyl vinyl ketone. Tetrahedron Lett. 2002, 43, 127–130. [Google Scholar] [CrossRef]
- CCDC number of the intermediate 3h is 836385. These data can be obtained free of charge from Cambridge Crystallographic Data Center via. Available online: http://www.ccdc.cam.ac.uk/datarequest/cif.
- Petrov, K.G.; Zhang, Y.; Carter, M.; Cockerill, G.S.; Dickerson, S.; Gauthier, C.A.; Guo, Y.; Mook, R.A.; Rusnak, D.W.; Walker, A.L.; et al. Optimization and SAR for dual ErbB-1/ErbB-2 tyrosine kinase inhibition in the 6-furanylquinazoline series. Bioorg. Med. Chem. Lett. 2006, 16, 4686–4691. [Google Scholar]
- Dishington, A.; Fillery, S.; Finalay, M.R.V. A one-pot sulfide to sulfone oxidation with m-chloroperoxybenzoic acid and sodium permanganate. Tetrahedron Lett. 2010, 51, 4211–4213. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ramesh, C.; Lei, P.-M.; Kavala, V.; Kuo, C.-W.; Yao, C.-F. A Convenient One-Pot Preparation of 2-Methyl-3-(phenylthio- methyl)quinolines from Morita-Baylis-Hillman Adducts and Their Oxidation to the Corresponding Sulfones. Molecules 2012, 17, 5081-5094. https://doi.org/10.3390/molecules17055081
Ramesh C, Lei P-M, Kavala V, Kuo C-W, Yao C-F. A Convenient One-Pot Preparation of 2-Methyl-3-(phenylthio- methyl)quinolines from Morita-Baylis-Hillman Adducts and Their Oxidation to the Corresponding Sulfones. Molecules. 2012; 17(5):5081-5094. https://doi.org/10.3390/molecules17055081
Chicago/Turabian StyleRamesh, Chintakunta, Po-Min Lei, Veerababurao Kavala, Chun-Wei Kuo, and Ching-Fa Yao. 2012. "A Convenient One-Pot Preparation of 2-Methyl-3-(phenylthio- methyl)quinolines from Morita-Baylis-Hillman Adducts and Their Oxidation to the Corresponding Sulfones" Molecules 17, no. 5: 5081-5094. https://doi.org/10.3390/molecules17055081
APA StyleRamesh, C., Lei, P.-M., Kavala, V., Kuo, C.-W., & Yao, C.-F. (2012). A Convenient One-Pot Preparation of 2-Methyl-3-(phenylthio- methyl)quinolines from Morita-Baylis-Hillman Adducts and Their Oxidation to the Corresponding Sulfones. Molecules, 17(5), 5081-5094. https://doi.org/10.3390/molecules17055081





