Parallel Synthesis of Peptide-Like Macrocycles Containing Imidazole-4,5-dicarboxylic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Characterization
3. Experimental
3.1. General
3.2. LC-MS Analysis
3.3. NMR Spectroscopy
3.4. X-ray Crystallography
3.5. Molecular Modeling
3.6. Synthetic Procedures
4. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- Walsh, C.T. The chemical versatility of natural-product assembly lines. Acc. Chem. Res. 2008, 41, 4–10. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. Natural products from marine organisms and their associated microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, W.C.; Battershill, C.N.; Liptrot, C.H.; Cobb, R.E.; Bourne, D.G.; Jaspars, M.; Long, P.F.; Newman, D.J. Biomedicinals from the phytosymbionts of marine invertebrates: A molecular approach. Methods 2007, 42, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.-H.; Zhou, J. Plant cyclopeptides. Chem. Rev. 2006, 106, 840–895. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Holly, F.W.; Nutt, R.F.; Bergstrand, S.J.; Brady, S.F.; Hirschmann, R.; Glitzer, M.S.; Saperstein, R. Highly active cyclic and bicyclic somatostatin analogues of reduced ring size. Nature 1979, 280, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Freidlinger, R.M.; Perlow, D.S.; Paleveda, W.J., Jr.; Holly, F.W.; Stachan, R.G.; Nutt, R.F.; Arison, B.H.; Homnick, C.; Randall, W.C.; et al. A potent cyclic hexapeptide analogue of somatostatin. Nature 1981, 292, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Vagner, J.; Qu, H.; Hruby, V.J. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Janin, Y.L. Peptides with anticancer use or potential. Amino Acids 2003, 25, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.J.; Faulkner, D.J. Bistratamides E–J, modified cyclic hexapeptides from the Philippines ascidian Lissoclinum bistratum. J. Nat. Prod. 2003, 66, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.; Moody, C.J. From amino acids to heteroaromatics—Thiopeptide antibiotics, nature’s heterocyclic peptides. Angew. Chem. Int. Ed. Engl. 2007, 46, 7930–7954. [Google Scholar] [CrossRef] [PubMed]
- Bertram, A.; Blake, A.J.; de Turiso, F.G.-L.; Hannam, J.S.; Jolliffe, K.A.; Pattenden, G.; Skae, M. Concise synthesis of stereodefined, thiazole-containing cyclic hexa- and octapeptide relatives of the Lissoclinums, via cyclooligomerisation reactions. Tetrahedron 2003, 59, 6979–6990. [Google Scholar] [CrossRef]
- Bertram, A.; Maulucci, N.; New, O.M.; Nor, S.M.M.; Pattenden, G. Synthesis of libraries of thiazole, oxazole, and imidazole-based cyclic peptides from azole-based amino acids. A new synthetic approach to Bistratamides and Didmolamides. Org. Biomol. Chem. 2007, 5, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Haberhauer, G.; Drosdow, E.; Oeser, T.; Rominger, F. Structural investigation of Westiellamide analogues. Tetrahedron 2008, 64, 1853–1859. [Google Scholar] [CrossRef]
- Ying, Y.; Taori, K.; Kim, H.; Hong, J.; Luesch, H. Total synthesis and molecular target of Largazole, a histone deacetylase inhibitor. J. Am. Chem. Soc. 2008, 130, 8455–8459. [Google Scholar] [CrossRef] [PubMed]
- You, S.-L.; Kelly, J.W. Highly efficient biomimetic total synthesis and structural verification of Bistratamides E and J from Lissoclinum bistratum. Chem. Eur. J. 2004, 10, 71–75. [Google Scholar] [CrossRef] [PubMed]
- You, S.-L.; Deechongkit, S.; Kelly, J.W. Solid-phase synthesis and stereochemical assignments of Tenuecyclamides A–D employing heterocyclic amino acids derived from commercially available Fmoc α-amino acids. Org. Lett. 2004, 6, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Abo-Ghalia, M.; Amr, A. Synthesis and investigation of a new cyclo(Na-dipicolinyl)pentapeptide of a breast and CNS cytotoxic activity and an ionophoric specificity. Amino Acids 2004, 26, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.; de Cuba, K.R.; Calvet-Vitale, S.; Geenevasen, J.A.J.; Hermkens, P.H.H.; Hiemstra, H.; van Maarseveen, J.H. Backbone amide linker strategy for the synthesis of 1,4-triazole-containing cyclic tetra- and pentapeptides. Eur. J. Org. Chem. 2008, 2008, 2592–2600. [Google Scholar] [CrossRef]
- Chen, W.; Yang, J.Z.; Andersen, R.; Nielsen, L.H.; Borchardt, R.T. Evaluation of the permeation characteristics of a model opioid peptide, H-Tyr-D-Ala-Gly-Phe-D-Leu-OH (DADLE), and its cyclic prodrugs across the blood-brain barrier using an in situ perfused rat brain model. J. Pharmacol. Exp. Ther. 2002, 303, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.; Ovadia, O.; Shalev, D.E.; Senderovich, H.; Qadri, B.; Yehezkel, T.; Salitra, Y.; Sheynis, T.; Jelinek, R.; Gilon, C.; et al. Effect of structural and conformational modifications, including backbone cyclization, of hydrophilic hexapeptides on their intestinal permeability and enzymatic stability. J. Med. Chem. 2007, 50, 6201–6211. [Google Scholar] [CrossRef] [PubMed]
- Liederer, B.M.; Phan, K.T.; Ouyang, H.; Borchardt, R.T. Significant differences in the disposition of cyclic prodrugs of opioid peptides in rats and guinea pigs following IV administration. J. Pharm. Sci. 2005, 94, 2676–2687. [Google Scholar] [CrossRef] [PubMed]
- Liederer, B.M.; Fuchs, T.; Vander Velde, D.; Siahaan, T.J.; Borchardt, R.T. Effects of amino acid chirality and the chemical linker on the cell permeation characteristics of cyclic prodrugs of opioid peptides. J. Med. Chem. 2006, 49, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Linde, Y.; Ovadia, O.; Safrai, E.; Xiang, Z.; Portillo, F.P.; Shalev, D.E.; Haskell-Luevano, C.; Hoffman, A.; Gilon, C. Structure-activity relationship and metabolic stability studies of backbone cyclization and N-methylation of melanocortin peptides. Biopolymers 2008, 90, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Malakoutikhah, M.; Teixidó, M.; Giralt, E. Toward an optimal blood-brain barrier shuttle by synthesis and evaluation of peptide libraries. J. Med. Chem. 2008, 51, 4881–4889. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Andersen, T.E.; Chen, W.; Nofsinger, R.; Steffansen, B.; Borchardt, R.T. A comparison of the effects of P-glycoprotein inhibitors on the blood-brain barrier permeation of cyclic prodrugs of an opioid peptide (DADLE). J. Pharm. Sci. 2009, 98, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Rezai, T.; Yu, B.; Millhauser, G.L.; Jacobson, M.P.; Lokey, R.S. Testing the conformational hypothesis of passive membrane permeability using synthetic cyclic peptide diastereomers. J. Am. Chem. Soc. 2006, 128, 2510–2511. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liao, W.; Arora, P.S. Enhanced metabolic stability and protein-binding properties of artificial α-helices derived from a hydrogen-bond surrogate: Application to Bcl-xL. Angew. Chem. Int. Ed. Engl. 2005, 44, 6525–6529. [Google Scholar] [CrossRef] [PubMed]
- Terrett, N.K. Methods for the synthesis of macrocycle libraries for drug discovery. Drug Discov. Today Technol. 2010, 7, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Llobet, A.; Murillo, T.; Bello, P.; Cilibrizzi, A.; Hodgkinson, J.T.; Galloway, W.R.J.D.; Bender, A.; Welch, M.; Spring, D.R. Diversity-oriented synthesis of macrocyclic peptidomimetics. Proc. Natl. Acad. Sci. USA 2011, 108, 6793–6798. [Google Scholar] [CrossRef] [PubMed]
- Baures, P.W. Imidazole-4,5-dicarboxylic acid: A versatile scaffold for drug discovery and materials research. Trends Heterocycl. Chem. 2006, 11, 1–22. [Google Scholar] [CrossRef]
- Xu, Z.; DiCesare, J.C.; Baures, P.W. Parallel synthesis of an oligomeric imidazole-4,5-dicarboxamide library. J. Comb. Chem. 2010, 12, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Solinas, R.; DiCesare, J.C.; Baures, P.W. Parallel synthesis of a library of symmetrically and disymmetrically-disubstituted imidazole-4,5-dicarboxamides bearing amino acid esters. Molecules 2009, 14, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Solinas, R.; DiCesare, J.C.; Baures, P.W. Parallel synthesis of an imidazole-4,5-dicarboxylic acid library bearing amino acid esters and alkanamines. Molecules 2008, 13, 3149–3170. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombarto, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.L.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule of five revolution. Drug Discov. Today 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- de Groot, F.M.H.; Loos, W.J.; Koekkoek, R.; van Berkom, L.W.A.; Busscher, G.F.; Seelen, A.E.; Albrecht, C.; de Bruijn, P.; Scheeren, H.W. Elongated multiple electronic cascade and cyclization spacer systems in activatible anticancer prodrugs for enhanced drug release. J. Org. Chem. 2001, 66, 8815–8830. [Google Scholar] [CrossRef] [PubMed]
- Marugan, J.J.; Zheng, W.; Motabar, O.; Southall, N.; Goldin, E.; Westbroek, W.; Stubblefield, B.K.; Sidransky, E.; Aungst, R.A.; Lea, W.A.; et al. Evaluation of quinazoline analogues as glucocerebrosidase inhibitors with chaperone activity. J. Med. Chem. 2011, 54, 1033–1058. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-M.; Yang, W.-B.; Kuo, T.-H.; Tsai, K.-C.; Lin, C.-H.; Yang, A.-S.; Liang, P.-H.; Wong, C.-H. Design, synthesis, and evaluation of trifluoromethyl ketones as inhibitors of SARS-CoV 3CL protease. Bioorg. Med. Chem. 2008, 16, 4652–4660. [Google Scholar] [CrossRef] [PubMed]
- Spartan’06. Wavefunction, Inc.: Irvine, CA, USA, 2006.
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.R.; Sandstrom, S.L.; Yang, J.; Davis, R.; Prakash, O.; Baures, P.W. Intramolecular hydrogen bond strength and pKa determination of N,N′-disubstituted imidazole-4,5-dicarboxamides. Org. Lett. 2005, 7, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Baures, P.W.; Rush, J.R.; Wiznycia, A.V.; Desper, J.; Helfrich, B.A.; Beatty, A.M. Intramolecular hydrogen bonding and intermolecular dimerization in the crystal structures of imidazole-4,5-dicarboxylic acid derivatives. Cryst. Growth Des. 2002, 2, 653–664. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-93; University of Göttingen: Göttingen, Germany, 1993. [Google Scholar]
Sample Availability: Contact the authors. |

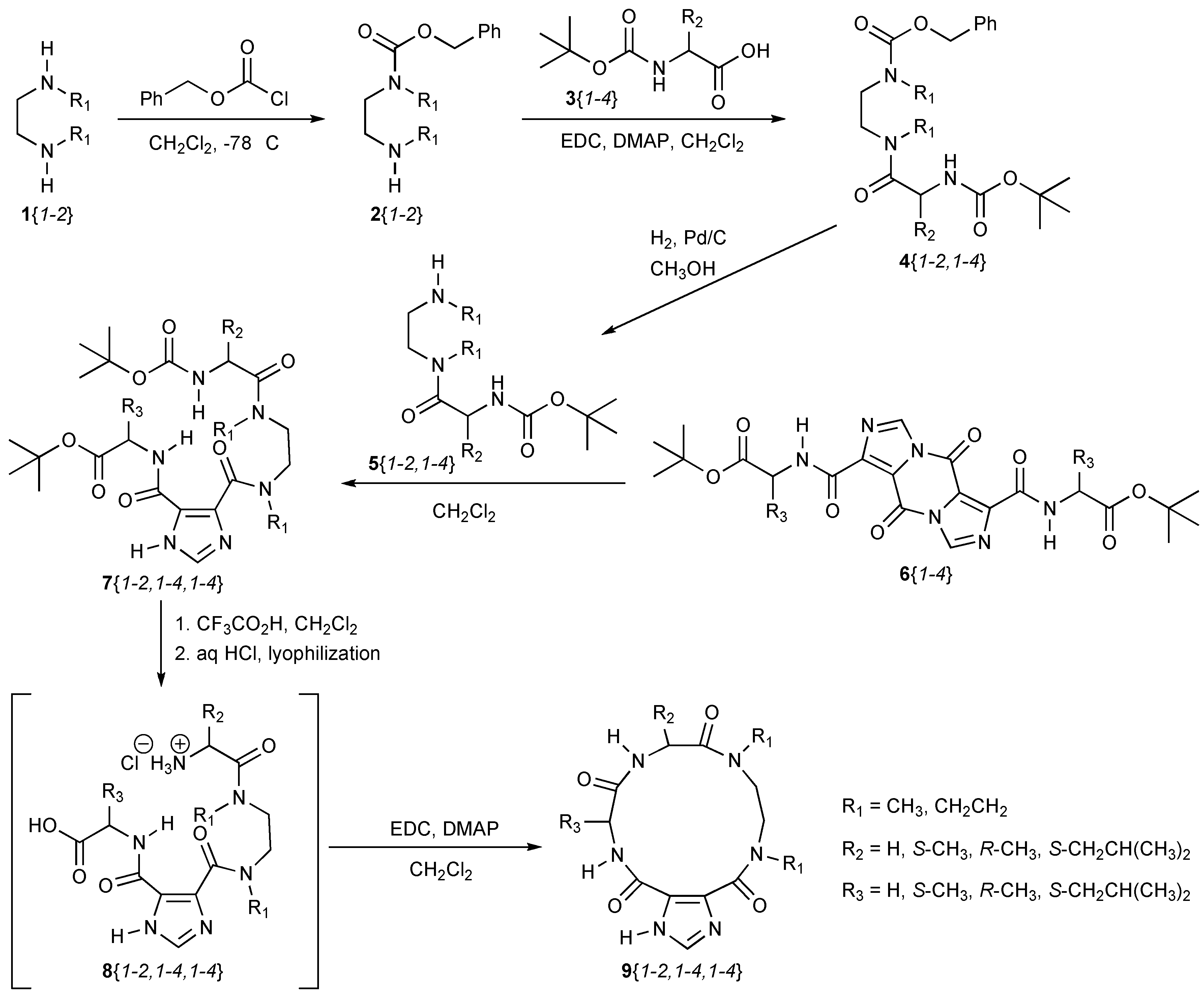

| R1 = Me | ||||
| R2 = H | R2 = (S)-CH3 | R2 = (R)-CH3 | R2 = (S)-CH2CH(CH3)2 | |
| R3 = H | 9{1,1,1}; 7% | 9{1,2,1}; — | 9{1,3,1}; — | 9{1,4,1}; — |
| R3 = (S)-CH3 | 9{1,1,2}; 7% | 9{1,2,2}; 32% | 9{1,3,2}; 22% | 9{1,4,2}; 14% |
| R3 = (R)-CH3 | 9{1,1,3}; 17% | 9{1,2,3}; — | 9{1,3,3}; — | 9{1,4,3}; 37% |
| R3 = (S)-CH2CH(CH3)2 | 9{1,1,4}; 37% | 9{1,2,4}; 12% | 9{1,3,4}; 11% | 9{1,4,4}; ‡ |
| R1 = Et | ||||
| R2 = H | R2 = (S)-CH3 | R2 = (R)-CH3 | R2 = (S)-CH2CH(CH3)2 | |
| R3 = H | 9{2,1,1}; — | 9{2,2,1}; — | 9{2,3,1}; 3% | 9{2,4,1}; 8% |
| R3 = (S)-CH3 | 9{2,1,2}; 9% | 9{2,2,2}; 7% | 9{2,3,2}; 10% | 9{2,4,2}; 11% |
| R3 = (R)-CH3 | 9{2,1,3}; 8% | 9{2,2,3}; 8% | 9{2,3,3}; 9% | 9{2,4,3}; — |
| R3 = (S)-CH2CH(CH3)2 | 9{2,1,4}; ‡ | 9{2,2,4}; ‡ | 9{2,3,4}; ‡ | 9{2,4,4}; — |
| cmpd | MW (g/mol) | cLogP | cmpd | MW (g/mol) | cLogP | |
|---|---|---|---|---|---|---|
| 9{1,1,1} | 322.32 | −1.70 | 9{2,1,1} | 350.37 | −0.64 | |
| 9{1,1,2} | 336.35 | −1.36 | 9{2,1,2} | 364.40 | −0.12 | |
| 9{1,1,3} | 336.35 | −1.36 | 9{2,1,3} | 364.40 | −0.12 | |
| 9{1,1,4} | 378.43 | 0.10 | 9{2,1,4} | 406.40 | 1.33 | |
| 9{1,2,1} | 336.35 | −1.36 | 9{2,2,1} | 364.40 | −0.12 | |
| 9{1,2,2} | 350.37 | −0.84 | 9{2,2,2} | 378.43 | 0.40 | |
| 9{1,2,3} | 350.37 | −0.84 | 9{2,2,3} | 378.43 | 0.40 | |
| 9{1,2,4} | 392.45 | 0.62 | 9{2,2,4} | 420.51 | 1.85 | |
| 9{1,3,1} | 336.35 | −1.36 | 9{2,3,1} | 364.40 | −0.12 | |
| 9{1,3,2} | 350.37 | −0.84 | 9{2,3,2} | 378.43 | 0.40 | |
| 9{1,3,3} | 350.37 | −0.84 | 9{2,3,3} | 378.43 | 0.40 | |
| 9{1,3,4} | 392.45 | 1.85 | 9{2,3,4} | 420.51 | 1.85 | |
| 9{1,4,1} | 378.43 | 0.10 | 9{2,4,1} | 406.48 | 1.33 | |
| 9{1,4,2} | 392.45 | 0.62 | 9{2,4,2} | 420.51 | 1.85 | |
| 9{1,4,3} | 434.53 | 0.62 | 9{2,4,3} | 420.51 | 1.85 | |
| 9{1,4,4} | 434.53 | 2.08 | 9{2,4,4} | 462.59 | 3.31 |
| Crystal data | 9{2,4,1} | 9{2,4,3} | 9{2,1,3} |
|---|---|---|---|
| formula | C19H30N6O4 + 0.16O | C20H32N6O4 | C16H24N6O4 + H2O |
| weight (g mol−1) | 409.03 | 420.51 | 382.43 |
| crystal size (mm) | 0.45 × 0.38 × 0.28 | 0.45 × 0.13 × 0.07 | 0.35 × 0.07 × 0.05 |
| crystal system | monoclinic | monoclinic | orthorhombic |
| space group | P21 | P21 | P212121 |
| a (Å) | 13.7441(2) | 14.0826(7) | 8.6170(1) |
| b (Å) | 14.4322(2) | 13.9531(7) | 17.9394(3) |
| c (Å) | 21.3948(3) | 22.4144(12) | 24.4406(4) |
| β (°) | 94.192(1) | 96.766(3) | - |
| cell volume (Å3) | 4,232.5(1) | 4,373.7(4) | 3,778.1(1) |
| Z | 8 | 8 | 8 |
| temp (K) | 100(2) | 100(2) | 100(2) |
| S | 1.017 | 1.093 | 0.994 |

| Compound | ||||||
|---|---|---|---|---|---|---|
| Torsion Angle | 9{2,4,1} ‡ | 9{2,4,3} ‡ | 9{2,1,3} † | Minimized 9{2,4,1} § | ||
| 1-2-3-4 | 131.2 ± 4.3° | 138.8 ± 6.4° | 134.1 ±4.0° | 109.6° | ||
| 2-3-4-5 | −60.3 ± 13.8° | −66.3 ± 3.0° | −77.4 ± 0.3° | −82.8° | ||
| 3-4-5-6 | 164.2 ± 4.5° | 165.5 ± 5.2° | 165.0 ± 1.6° | 156.0° | ||
| 4-5-6-7 | −108.4 ± 12.5° | −109.0 ± 6.8° | −95.2 ± 1.7° | −93.7° | ||
| 5-6-7-8 | 106.1 ± 7.6° | 111.1 ± 9.1° | 112.0 ± 5.7° | 129.1° | ||
| 6-7-8-9 | 175.4 ± 3.2° | 177.1 ± 2.3° | 174.7 ± 1.8° | −176.5° | ||
| 7-8-9-10 | 102.2 ± 1.7° | 100.8 ± 5.4° | 98.5 ± 3.9° | 95.5° | ||
| 8-9-10-11 | −75.6 ± 4.2° | −77.0 ± 4.2° | −71.9 ± 0.9° | −89.4° | ||
| 9-10-11-12 | 96.3 ± 0.9° | 99.4 ± 2.9° | 103.6 ± 3.1° | 90.5° | ||
| 10-11-12-13 | 176.1 ± 1.0° | 175.4 ± 3.0° | 173.3 ± 0.2° | −175.0° | ||
| 11-12-13-14 | 147.2 ± 3.9° | 145.9 ± 3.1° | 137.3 ± 1.6° | 149.8° | ||
| 12-13-14-1 | −2.2 ± 1.2° | −3.8 ± 1.9° | −1.1 ± 4.1° | −2.6° | ||
| 13-14-1-2 | 15.7 ± 4.5° | 13.6 ± 4.3° | 16.3 ± 0.9° | 39.9° | ||
| 14-1-2-3 | −167.9 ± 2.1° | −166.9 ± 2.7° | −166.4 ± 0.3° | −163.5° | ||
| Measurement | Molecule in Asymmetric Unit | |||
|---|---|---|---|---|
| 9{2,4,1} | 9{2,4,3} | 9{2,1,3} | Minimized 9{2,4,1} | |
| N2…O(=C12) | 2.69 ± 0.02 Å | 2.72 ± 0.02 Å | 2.78 ± 0.01 Å | 2.74 Å |
| N2-H…O(=C12) | 166.8 ± 3.5° | 160.9 ± 2.6° | 161.7 ± 5.9° | 151.4° |
| C12=O…H(-N2) | 106.2 ± 0.8° | 106.4 ± 1.3° | 103.4 ± 2.1° | 114.4° |
| compound | Name |
|---|---|
| 9{1,1,1} | cyclo(N,N′dimethylethylenediamine-glycyl-glycyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,1,2} | cyclo(N,N′dimethylethylenediamine-glycyl-S-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,1,3} | cyclo(N,N′dimethylethylenediamine-glycyl-R-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,1,4} | cyclo(N,N′dimethylethylenediamine-glycyl-S-leucyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,2,1} | cyclo(N,N′dimethylethylenediamine-S-alanyl-glycyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,2,2} | cyclo(N,N′dimethylethylenediamine-S-alanyl-S-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,2,3} | cyclo(N,N′dimethylethylenediamine-S-alanyl-R-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,2,4} | cyclo(N,N′dimethylethylenediamine-S-alanyl-S-leucyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,3,1} | cyclo(N,N′dimethylethylenediamine-R-alanyl-glycyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,3,2} | cyclo(N,N′dimethylethylenediamine-R-alanyl-S-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,3,3} | cyclo(N,N′dimethylethylenediamine-R-alanyl-R-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,3,4} | cyclo(N,N′dimethylethylenediamine-R-alanyl-S-leucyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,4,1} | cyclo(N,N′dimethylethylenediamine-S-leucyl-glycyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,4,2} | cyclo(N,N′dimethylethylenediamine-S-leucyl-S-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,4,3} | cyclo(N,N′dimethylethylenediamine-S-leucyl-R-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{1,4,4} | cyclo(N,N′dimethylethylenediamine-S-leucyl-S-leucyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,1,1} | cyclo(N,N′diethylethylenediamine-glycyl-glycyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,1,2} | cyclo(N,N′diethylethylenediamine-glycyl-S-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,1,3} | cyclo(N,N′diethylethylenediamine)-glycyl-R-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,1,4} | cyclo(N,N′diethylethylenediamine-glycyl-S-leucyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,2,1} | cyclo(N,N′diethylethylenediamine-S-alanyl-glycyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,2,2} | cyclo(N,N′diethylethylenediamine-S-alanyl-S-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,2,3} | cyclo(N,N′diethylethylenediamine-S-alanyl-R-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,2,4} | cyclo(N,N′diethylethylenediamine-S-alanyl-S-leucyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,3,1} | cyclo(N,N′diethylethylenediamine-R-alanyl-glycyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,3,2} | cyclo(N,N′diethylethylenediamine-R-alanyl-S-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,3,3} | cyclo(N,N′diethylethylenediamine-R-alanyl-R-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,3,4} | cyclo(N,N′diethylethylenediamine-R-alanyl-S-leucyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,4,1} | cyclo(N,N′diethylethylenediamine-S-leucyl-glycyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,4,2} | cyclo(N,N′diethylethylenediamine-S-leucyl-S-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,4,3} | cyclo(N,N′diethylethylenediamine-S-leucyl-R-alanyl-4,5-dicarbonyl)-1H-imidazole |
| 9{2,4,4} | cyclo(N,N′diethylethylenediamine-S-leucyl-S-leucyl-4,5-dicarbonyl)-1H-imidazole |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, Z.; Wheeler, K.A.; Baures, P.W. Parallel Synthesis of Peptide-Like Macrocycles Containing Imidazole-4,5-dicarboxylic Acid. Molecules 2012, 17, 5346-5362. https://doi.org/10.3390/molecules17055346
Xu Z, Wheeler KA, Baures PW. Parallel Synthesis of Peptide-Like Macrocycles Containing Imidazole-4,5-dicarboxylic Acid. Molecules. 2012; 17(5):5346-5362. https://doi.org/10.3390/molecules17055346
Chicago/Turabian StyleXu, Zhigang, Kraig A. Wheeler, and Paul W. Baures. 2012. "Parallel Synthesis of Peptide-Like Macrocycles Containing Imidazole-4,5-dicarboxylic Acid" Molecules 17, no. 5: 5346-5362. https://doi.org/10.3390/molecules17055346





