Maturation of Murine Bone Marrow-Derived Dendritic Cells Induced by Radix Glycyrrhizae Polysaccharide
Abstract
:1. Introduction
2. Results and Discussion
2.1. GP Induces Phenotypic Maturation of DCs

2.2. GP Inhibits the Endocytic Activity of DCs
2.3. GP-Treated DCs Enhance Allogenic T Cell Proliferation
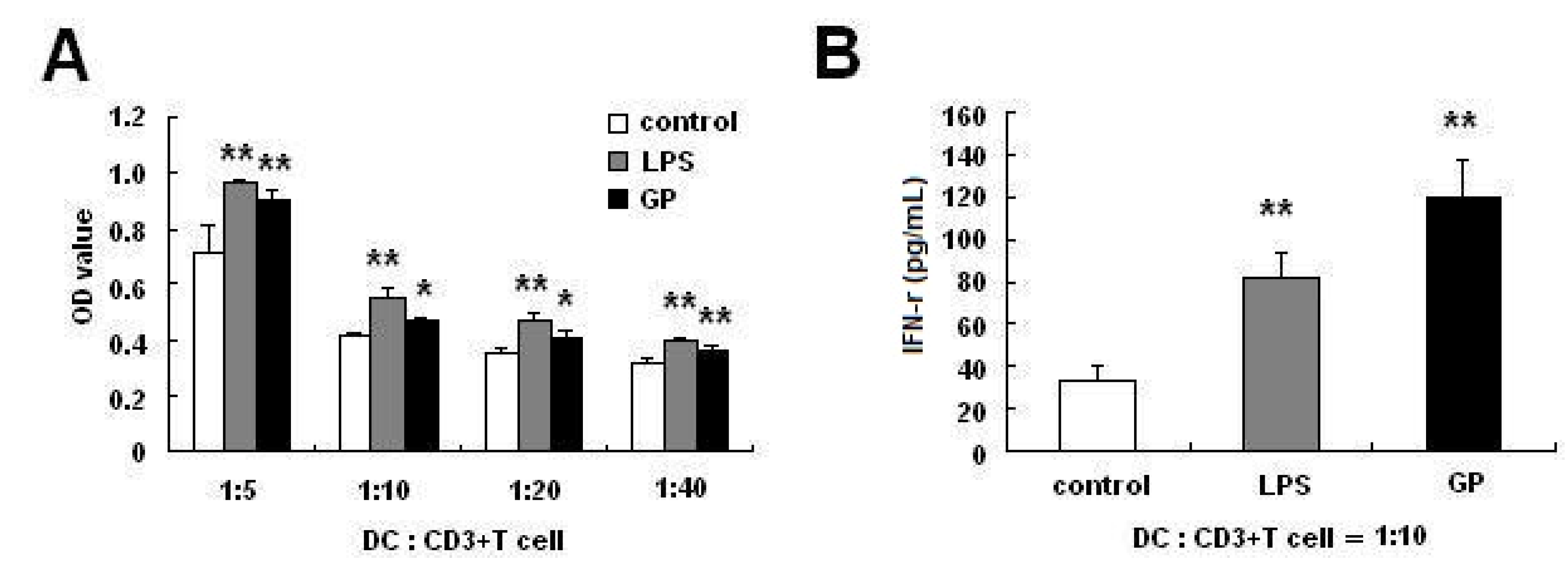
2.4. GP Enhances IL-12 Production in DCs
2.5. GP Induces IL-12 p70 Synthesis through TLR4 Signaling Pathways


2.6. Discussion
3. Experimental
3.1. Mice
3.2. GP and Other Chemicals
3.3. Induction of Mouse Bone Marrow-Derived DCs
3.4. Flow Cytometric Analysis
3.5. Endocytosis Assay
3.6. Cytokine Assays
3.7. Mixed Lymphocyte Reaction (MLR)
3.8. Real-Time PCR
3.9. Neutralization Experiments
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
- Samples Availability: Samples of the Radix Glycyrrhizae polysaccharide are available from the authors.
References and Notes
- Asada, Y.; Li, W.; Yoshikawa, T. Biosynthesis of the dimethylallyl moiety of glabrol in Glycyrrhiza glabra hairy root cultures via a non-mevalonate pathway. Phytochemistry 2000, 55, 323–326. [Google Scholar]
- Xu, H.; Fabricant, D.S.; Piersen, C.E.; Bolton, J.L.; Pezzuto, J.M.; Fong, H.; Totura, S.; Farnsworth, N.R.; Constantinou, A.I. A preliminary RAPD-PCR analysis of Cimicifuga species and other botanicals used for women’s health. Phytomedicine 2002, 9, 757–762. [Google Scholar] [CrossRef]
- Arase, Y.; Ikeda, K.; Murashima, N.; Chayama, K.; Tsubota, A.; Koida, I.; Suzuki, Y.; Saitoh, S.; Kobayashi, M.; Kumada, H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997, 79, 1494–1500. [Google Scholar] [CrossRef]
- Davis, E.A.; Morris, D.J. Medicinal uses of licorice through the millennia: The good and plenty of it. Mol. Cell. Endocrinol. 1991, 78, 1–6. [Google Scholar] [CrossRef]
- Takahara, T.; Watanabe, A.; Shiraki, K. Effects of glycyrrhizin on hepatitis B surface antigen: A biochemical and morphological study. J. Hepatol. 1994, 21, 601–609. [Google Scholar] [CrossRef]
- Yang, G.; Yu, Y. Immunopotentiating effect of traditional Chinese drugs—Ginsenoside and glycyrrhiza polysaccharide (in Chinese). Proc. Chin. Acad. Med. Sci. Peking Union Med. Coll. 1990, 5, 188–193. [Google Scholar]
- Nose, M.; Terawaki, K.; Oguri, K.; Ogihara, Y.; Yoshimatsu, K.; Shimomura, K. Activation of macrophages by crude polysaccharide fractions obtained from shoots of Glycyrrhiza glabra and hairy roots of Glycyrrhiza uralensis in vitro. Biol. Pharm. Bull. 1998, 21, 1110–1112. [Google Scholar] [CrossRef]
- Takada, K.; Tomoda, M.; Shimizu, N. Core structure of glycyrrhizan GA, the main polysaccharide from the stolon of Glycyrrhiza glabra var. glandulifera; anti-complementary and alkaline phosphatase-inducing activities of the polysaccharide and its degradation products. Chem. Pharm. Bull. (Tokyo) 1992, 40, 2487–2490. [Google Scholar]
- Wang, Y.W.; Zhang, H.B.; Lv, J.; Shi, Y.R.; He, M.; Wang, S.S. Inhibition of Glycyrrhiza polysaccharides on virus (in Chinese). Acta Sci. Nat. Univ. Nankaiensis 2000, 33, 46–48. [Google Scholar]
- Wang, C.; Xie, G.R.; Shi, Y.R.; Zhang, L.H. Study on the anti-tumor effect in vivo of glycyrrhizia polysaccharide and its mechanism (in Chinese). Chin. Clin. Oncol. 2003, 8, 85–87. [Google Scholar]
- Crowley, M.; Inaba, K.; Steinman, R.M. Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins. J. Exp. Med. 1990, 172, 383–386. [Google Scholar] [CrossRef]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Ardeshna, K.M.; Pizzey, A.R.; Devereux, S.; Khwaja, A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cell. Blood 2000, 96, 1039–1046. [Google Scholar]
- Sallusto, F.; Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef]
- Sallusto, F.; Cella, M.; Danieli, C.; Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: Downregulation by cytokines and bacterial products. J. Exp. Med. 1995, 182, 389–400. [Google Scholar] [CrossRef]
- Roake, J.A.; Rao, A.S.; Morris, P.J.; Larsen, C.P.; Hankins, D.F.; Austyn, J.M. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 1995, 181, 2237–2247. [Google Scholar] [CrossRef]
- Cella, M.; Engering, A.; Pinet, V.; Pieters, J.; Lanzavecchia, A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 1997, 388, 782–787. [Google Scholar]
- de Jong, E.C.; Smits, H.H.; Kapsenberg, M.L. Dendritic cell-mediated T cell polarization. Springer Semin. Immunopathol. 2005, 26, 289–307. [Google Scholar] [CrossRef]
- Yoshimura, S.; Bondeson, J.; Foxwell, B.M.; Brennan, F.M.; Feldmann, M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: Coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int. Immunol. 2001, 13, 675–683. [Google Scholar] [CrossRef]
- Ardeshna, K.M.; Pizzey, A.R.; Devereux, S.; Khwaja, A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 2000, 96, 1039–1046. [Google Scholar]
- Lin, Y.L.; Liang, Y.C.; Lee, S.S.; Chiang, B.L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 1–10. [Google Scholar] [CrossRef]
- Kim, G.Y.; Han, M.G.; Song, Y.S.; Shin, B.C.; Shin, Y.I.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Kwak, J.Y.; Bae, Y.S.; et al. Proteoglycan isolated from Phellinus linteus induces toll-like receptors 2- and 4-mediated maturation of murine dendritic cells via activation of ERK, p38, and NF-kappaB. Biol. Pharm. Bull. 2004, 27, 1656–1662. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fitz, L.; Ryan, M.; Hewick, R.M.; Clark, S.C.; Chan, S.; Loudon, R.; Sherman, F.; Perussia, B.; Trinchieri, G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989, 170, 827–845. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, F.; Jin, Z.; Wang, J.; Xu, X. Nitrite oxide and inducible nitric oxide synthase were regulated by polysaccharides isolated from Glycyrrhiza uralensis Fisch. J. Ethnopharmacol. 2008, 118, 59–64. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, F.; Wang, J.; Jin, Z.; Xu, X. Macrophage immunomodulatory activity of polysaccharides isolated from Glycyrrhiza uralensis fish. Int. Immunopharmacol. 2008, 8, 43–50. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Liu, B.; Xu, L.; Zhao, H.; Lu, A. Down-regulation of treg cells and up-regulation of TH1/TH2 cytokine ratio were induced by polysaccharide from radix glycyrrhizae in h22 hepatocarcinoma bearing mice. Molecules 2011, 16, 8343–8352. [Google Scholar] [CrossRef]
- Seder, R.A.; Gazzinelli, R.; Sher, A.; Paul, W.E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA 1993, 90, 10188–10192. [Google Scholar]
- Rhee, S.H.; Hwang, D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappaB and expression of the inducible cyclooxygenase. J. Biol. Chem. 2000, 275, 34035–34040. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Palsson-McDermott, E.M.; Bowie, A.G.; Jefferies, C.A.; Mansell, A.S.; Brady, G.; Brint, E.; Dunne, A.; Gray, P.; Harte, M.T.; et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 2001, 413, 78–83. [Google Scholar] [CrossRef]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–702. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.; He, X.; Liu, B.; Xu, L.; Lu, C.; Zhao, H.; Niu, X.; Chen, S.; Lu, A. Maturation of Murine Bone Marrow-Derived Dendritic Cells Induced by Radix Glycyrrhizae Polysaccharide. Molecules 2012, 17, 6557-6568. https://doi.org/10.3390/molecules17066557
Li X, He X, Liu B, Xu L, Lu C, Zhao H, Niu X, Chen S, Lu A. Maturation of Murine Bone Marrow-Derived Dendritic Cells Induced by Radix Glycyrrhizae Polysaccharide. Molecules. 2012; 17(6):6557-6568. https://doi.org/10.3390/molecules17066557
Chicago/Turabian StyleLi, Xiaobing, Xiaojuan He, Biao Liu, Li Xu, Cheng Lu, Hongyan Zhao, Xuyan Niu, Shilin Chen, and Aiping Lu. 2012. "Maturation of Murine Bone Marrow-Derived Dendritic Cells Induced by Radix Glycyrrhizae Polysaccharide" Molecules 17, no. 6: 6557-6568. https://doi.org/10.3390/molecules17066557



