3. Experimental
3.1. General Methods
Reagents, unless otherwise mentioned, were purchased from Aldrich and used without further purification. 1,2-Cyclohexanedione was purchased from Acros, Tetrahydrofuran (THF) and diethyl ether (Et2O) were distilled from sodium/benzophenone under argon at atmospheric pressure immediately prior to use. Toluene was distilled from calcium hydride under an inert atmosphere and stored over sodium. All other solvents were analytically pure grade and were used without further purification. Analytical and preparative HPLC were performed on a Waters HPLC system equipped with a 717-Plus autosampler, a 600-controller pump, a 996-photodiode array detector and a Gilson 202 fraction collector; the system was operated with the Millennium software (Waters). Selected wavelengths for chromatograms were 220 nm and 254 nm. Mobile phases were (A) H2O (0.1% TFA) and (B) MeCN (0.08% TFA). Separation conditions were as follows: Analytical: column C18 Vydac-218TP-54, gradient H2O/MeCN, Method A: 3 min [100/0], 3–20 min [50/50], 20–30 min [0/100], 30–40 min [0/100], 41 min [100/0]; flow, 1 mL/min. column Chromolith Performance RP-18e 100–4.6 mm, gradient H2O/MeCN, Method B: 1 min [100/0], 1–8 min [0/100], 8–11 min [0/100], 11.1 min [100/0]; flow, 6 mL/min. Preparative: column C18 Vydac-218TP-101510 or column C18 Phenomenex 58 × 21.2 × 10 mm, gradient H2O/MeCN. Method D: 3 min [100/0], 3–25 min [50/50], 25–35 min [0/100], 35–50 min [0/100], 51 min [0/100]; flow, 15 mL/min. Method E: 18 min [100/0], 18–40 min [50/50], 40–50 min [0/100], 50–65 min [0/100], 66 min [0/100]; flow, 15 mL/min. Method F: 3 min [100/0], 3–20 min [50/50], 20–30 min [0/100], 30–60 min [0/100], 61 min [0/100]; flow, 15 mL/min.
Preparative normal phase HPLC were performed on a Waters HPLC prep 4000 system equipped with a 4000-controller pump, a 486-absorbance detector with option to one wavelength, manual injector and Gilson 202 fraction collector. Mobile phases were (A) hexane and (B) EtOAc. Separation conditions are as follows: Preparative: column GL Sciences Inc-2GI95001 Inertsil PREP-SIL µm 50 × 250 mm, manual gradient hexane/EtOAc, Method G: 100% hexane until 2:1 hexane/EtOAc (manual change); flow, 70 mL/min.
Microwave irradiations were carried out with a professional “Initiator” microwave from Biotage at pre-fixed temperature. The intensity differed from 0 to 300W at frequency of 2.456 GHz.
1H and 13C-NMR spectra were recorded on Bruker DPX-300 and Avance DMX-600 spectrometers. 1H-NMR data was obtained by 2D, COSY and HOHAHA methods (t = 40 ms). 13C-NMR data was obtained by 2D techniques, NOESY, HMQC and HMBC methods with a delay of 3.45 60 ms respectively in the reverse mode. Chemical shifts are in ppm relative to TMS internal standard or relative to the residual solvent resonance.
Mass spectra analyses were recorded on an AUTOSPEC-FISSONS VG (Micromass) high-resolution mass spectrometer under DCI (desorption chemical ionization) conditions (CH4) and by ESI (electron spray ionization) mass spectrometry on a Q-TOF (quadropole time of flight) low-resolution micromass spectrometer (Micromass-Waters, Corp.). Microwave reactions were performed using a mono-mode Initiator station from Biotage. Purity of compounds was over 95% as assessed by two different HPLC conditions, QTOF-MS-MS was used for assessing purity of the molecular peaks.
3.2. Synthesis of Intermediates
Preparation of 1,4-dioxaspiro[4.5]decan-6-one (1): A solution of 1,2-cyclohexanedione (10 g, 0.089 mol) in toluene (300 mL), an equimolar amount of ethylene glycol (5 mL, 0.089 mol) and p-toluenesulfonic acid (100 mg) were heated at reflux for 8 h in a Dean-Stark apparatus (water was continuously separated). The resulting solution was washed twice with 1 N NaOH solution, dried over MgSO4 and concentrated under reduced pressure to afford 9.61 g (0.04 mol, 46% yield) of the crude yellow oily product. The crude product containing a certain amount of the diacetal 1′ (2:1 for the monoketone, according to NMR), was used without further purification in the next step. 1H-NMR (300 MHz, CDCl3) δ 3.93 (m, 4H, H-7', H-7"), 2.47 (m, 2H, H-6), 1.44–1.91 (m, 6H, H-3, H-4, H-5); 13C-NMR (75 MHz, CDCl3) δ 206.19 (C-1), 106.62 (C-2), 65.05 (C-7', C-7"), 39.51 (C-6), 36.77 (C-3), 26.07 (C-5), 22.55 (C-4); MS(CI+): 157 (MH+); 128 (M-CO). HRMS: m/z calc. for C8H12O3 (MH+) 157.0865; found 157.0835.
Preparation of 6-phenyl-1,4-dioxaspiro[4.5]decan-6-ol (2): To a solution of 1 containing 1 mmol of monoketone in dry THF (25 mL) at −78 °C under N2 was added dropwise a solution of phenylMgBr (2 mmol in 50 mL ether) The mixture was stirred at −78 °C and then was warmed to room temperature with stirring overnight. The reaction mixture was quenched by the addition of saturated aqueous NH4Cl. The organic layer was removed, and the aqueous layer was extracted twice with ether. The combined organic layers were washed with brine, dried over MgSO4, and concentrated under reduced pressure. The residue was purified by preparative normal phase-HPLC (method G, TLC hexane-EtOAc 2:1, vanillin) to give 4.27 g of the desired product as a white solid (60% yield). HPLC analysis Rt = 26.0 min (analytical method A); 1H-NMR (600 MHz, CDCl3) δ 7.57 (m, 2H, H-2'), 7.29 (m, 2H, H-3'), 7.24 (m, 1H, H-4'), 3.69 (m, 1H, Ha-7''), 3.64 (m, 1H, Ha-7'), 3.34 (m, 1H, Hb-7''), 2.83 (m, 1H, Hb-7'), 2.65 (bs, 1H, OH), 2.30 (m, 1H, Ha-3), 2.12 (m, 1H, Ha-6), 1.79 (m, 1H, Ha-4), 1.78 (m, 1H, Hb-3), 1.74 (m, 1H, Ha-5), 1.64 (m, 1H, Hb-5), 1.59 (m, 1H, Hb-6), 1.55 (m, 1H, Hb-4); 13C-NMR (150 MHz, CDCl3) δ 143.83 (C-1'), 127.22 (C-3'), 127.05 (C-2'), 126.68 (C-4'), 110.74 (C-1), 76.70 (C-2), 65.52 (C-7"), 65.09 (C-7'), 35.72 (C-3), 32.72 (C-6), 23.26 (C-5), 20.61 (C-4); MS(CI+): 234 (M+); 217 (M-OH+). HRMS: m/z calc. for C14H18O3 (M+) 234.1256; found 234.1240.
Preparation of 2-hydroxy-2-phenylcyclohexanone (3): Compound 2 (0.15 mmol) was treated with aqueous 80% TFA (3 mL) at 0 °C. The reaction mixture was stirred for 3 h at room temperature and then treated with aqueous saturated NaHCO3 solution at 0 °C until it became alkaline. The resulting mixture was extracted with CH2Cl2 (×2). The organic layer was washed with brine, dried over MgSO4, and concentrated under reduced pressure to give the desired product as a yellow oil (86–98% yield); HPLC analysis Rt = 22.8 min (analytical method A); 1H-NMR (300 MHz, CDCl3) δ 7.27 (m, 5H, Ph), 2.91 (m, 1H, Ha-6), 2.40 (m, 2H, Hb-6, Ha-3), 1.57–2.04 (m, 5H, Hb-3, H-4, H-5); 13C-NMR (75 MHz, CDCl3) δ 211.59 (C-1), 138.92 (C-1'), 128.12 (C-3'), 127.30 (C-4'), 125.37 (C-2'), 79.04 (C-2), 37.88 (C-6), 37.82 (C-3), 27.31 (C-5), 22.04 (C-4); MS(CI+): 190 (M+); 173 (M+-OH); 162 (M+-CO). HRMS: m/z calc. for C12H14O2 (M+) 190.0994; found 190.0984.
Preparation of 6-methyl-1,4-dioxaspiro[4.5]decan-6-ol (2′): Prepared similarly to 2, but using MeMgBr as Grignard reagent. The residue was purified by silica gel column chromatography (petroleum ether-Et2O 3:1 then, petroleum ether-Et2O-DCM 2:1:1, vanillin) to give 1.88 g of the desired product as a yellowish oil (60.5% yield). 1H-NMR (300 MHz, CDCl3) δ 4.00 (m, 4H, H-7', H-7"), 2.19 (bs, 1H, OH), 1.82 (m, 1H, Ha-3), 1.36–1.67 (m, 7H, Hb-3, H-6, H-5, H-4), 1.21 (s, 3H, Me); 13C-NMR (75 MHz, CDCl3) δ 111.13 (C-1), 73.91 (C-2), 65.60 (C-7"), 65.39 (C-7'), 37.85 (C-6), 31.57 (C-3), 23.32 (C-5), 22.49 (Me), 21.74 (C-4); MS(CI+): 172 (M+); 155 (M+-OH). HRMS: m/z calc. for C9H16O3 (M+) 172.1099; found 172.1084.
Preparation of 2-hydroxy-2-methylcyclohexanone (3′): Prepared similarly to 3 but starting from 2’. Yellow oil (82–91% yield); 1H-NMR (300 MHz, CDCl3) δ 3.83 (bs, 1H, OH), 2.54 (m, 2H, H-6), 2.11 (m, 2H, H-3), 1.61–1.85 (m, 4H, H-5, H-4), 1.41 (s, 3H, Me); 13C-NMR (75 MHz, CDCl3) δ 214.37 (C-1), 76.46 (C-2), 42.05 (C-6), 37.78 (C-3), 27.88 (C-5), 25.07 (Me), 23.01 (C-4); MS (CI+): 129 (MH+); 111 (M-OH). HRMS: m/z calc. for C7H12O2 (MH+) 129.0916; found 129.0928.
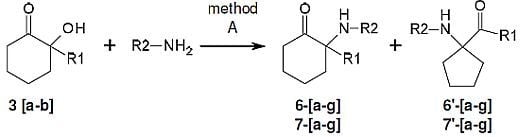
3.3. General Procedure for the Parallel Synthesis of Library Compounds 6a–g, 6′a–g
To seven pressure-tubes each containing 2-hydroxy-2-phenylcyclohexanone (3, 190 mg, 1 mmol) was added the appropriate primary amine (benzylamine, hexylamine, propylamine, isopropylamine, butylamine, aniline, 4-chloroaniline, 1.15 mmol). The tubes were flushed with argon, sealed and heated at 200 °C overnight in a Radley’s combinatorial station with stirring. The residues were diluted with ether and extracted with 0.1 M HCl. The acidic phase washed with ether and concentrated and the combined ether phase was dried over MgSO4 and concentrated. Preparative HPLC conditions were selected among methods D-F, according to the elution times obtained by analytical HPLC using method A.
3.4. Characterization Data for Compounds 6a–g, 6′a–g
For the NMR data numbering system of compounds
6a–g,
6′a–g please refer to
Table 5.
Table 5.
Numbering system for NMR chemical shift attribution of library compounds 6a–g, 6′a–g.
Table 5.
Numbering system for NMR chemical shift attribution of library compounds 6a–g, 6′a–g.
![Molecules 17 06784 i002]() |
Synthesis of 6a-6′a: benzylamine was used as amine component in the reaction. The mixture was purified by preparative HPLC using method D to give the desired products.
2-(Benzylamino)-2-phenylcyclohexanone (6a): White solid (36% yield); HPLC analysis Rt = 22.3 min (analytical method A); 1H-NMR (300 MHz, CDCl3 δ 7.54–7.67 (m, 2H, H-2′), 7.54–7.67 (m, 2H, H-3′), 7.54–7.67 (m, 1H, H-4′), 7.46–7.56 (m, 2H, H-3′′), 7.46–7.56 (m, 1H, H-4′′), 7.31–7.42 (m, 2H, H-2′′), 3.78 (d, 1H, CH2-Bn, 12.9), 3.74 (d, 1H, CH2-Bn, 12.9), 3.33 (ddd, 1H, Ha-3, 13.5, 5.7, 2.7), 2.54 (m, 2H, H-6), 2.08–2.22 (m, 1H, Hb-3), 2.08–2.22 (m, 1H, Ha-5), 1.96–2.00 (m, 1H, Ha-4), 1.73–1.88 (m, 1H, Hb-4), 1.73–1.88 (m, 1H, Hb-5); 13C-NMR (75 MHz, CD3OD) δ 206.96 (C-1), 132.43* (C-1′′), 131.98* (C-4′), 131.51* (C-1′), 131.29* (C-3′), 131.17* (C-3′′), 130.59* (C-4′′), 130.14* (C-2′′), 129.90* (C-2′), 74.28 (C-2), 47.90 (CH2-Bn), 40.47 (C-6), 34.38 (C-3), 28.74 (C-5), 22.80 (C-4); MS(CI+): 280 (MH+); 91 (C7H7+). HRMS: m/z calc. for C19H21NO (MH+) 280.1701; found 280.1734.
(1-(Benzylamino)cyclopentyl)(phenyl)methanone (6′a): White solid (12% yield); HPLC analysis Rt =23.7 min (analytical method A); 1H-NMR (600 MHz, CDCl3) δ 8.23 (m, 2H, H-2′), 7.43 (m, 2H, H-3′), 7.52 (m, 1H, H-4′), 7.21 (m, 2H, H-3′′), 7.19 (m, 2H, H-4′′), 7.05 (m, 2H, H-2′′), 3.49 (s, 2H, CH2-Bn), 2.40 (m, 2H, Ha-2), 1.90 (m, 2H, Hb-2), 1.80 (m, 2H, Ha-3), 1.69 (m, 2H, Hb-3); 13C-NMR (75 MHz, CDCl3) δ 204.30 (COPh), 140.24 (C-1′′), 136.28 (C-1′), 132.17 (C-4′), 129.33 (C-2′), 128.30 (C-3′′), 128.22 (C-2′′), 128.10 (C-3′), 126.99 (C-4′′), 75.17 (C-1), 49.32 (CH2-Bn), 36.68 (C-2), 24.66 (C-3); MS(CI+): 280 (MH+); 174 (M−COPh)+. HRMS: m/z calc. for C19H21NO (MH+) 280.1701; found 280.1726.
Synthesis of 6b-6′b: Hexylamine was used as amine component in the reaction. The mixture was purified by preparative HPLC using method D to give the desired products.
2-(Hexylamino)-2-phenylcyclohexanone (6b): Colorless oil (38% yield); HPLC analysis Rt = 25.3 min (analytical method A); 1H-NMR (300 MHz, CD3OD) δ 7.49–7.61 (m, 2H, H-2′), 7.49–7.61 (m, 2H, H-3′), 7.49–7.61 (m, 1H, H-4′), 3.29 (m, 2H, Ha-3), 2.72 (m, 1H, Ha-1′′), 2.47 (m, 1H, Hb-1′′), 2.47 (m, 2H, H-6), 1.96–2.16 (m, 1H, Hb-3), 1.96–2.16 (m, 1H, Ha-4), 1.96–2.16 (m, 1H, Ha-5), 1.82 (m, 1H, Hb-4), 1.82 (m, 1H, Hb-5), 1.59 (m, 2H, H-2′′), 1.28 (m, 2H, H-3′′), 1.28 (m, 2H, H-4′′), 1.28 (m, 2H, H-5′′), 0.88 (t, 3H, H-6′′, 6.6); 13C-NMR (75 MHz, CD3OD) δ 207.00 (C-1), 131.77 (C-4′), 131.49 (C-1′), 131.14 (C-3′), 129.59 (C-2′), 73.48 (C-2), 43.47 (C-1′′), 40.24 (C-6), 33.61 (C-3), 32.27 (C-2′′), 28.60 (C-5), 27.24 (C-3′′), 27.20 (C-4′′), 23.32 (C-5′′), 22.74 (C-4), 14.20 (C-6′′); MS(CI+): 274 (MH+); 245 (M-Et)+; 174 (M−NHHex)+. HRMS: m/z calc. for C18H27NO (MH+) 274.2170; found 274.2175.
(1-(Hexylamino)cyclopentyl)(phenyl)methanone (6'b): Yellowish oil (13% yield); HPLC analysis Rt = 26.3 min (analytical method A); 1H-NMR (300 MHz, CD3OD) δ 7.86 (m, 2H, H-2′), 7.66 (m, 1H, H-4′), 7.55 (m, 2H, H-3′), 2.96 (m, 2H, H-1′′), 2.61 (m, 2H, Ha-2), 2.15 (m, 2H, Hb-2), 2.15 (m, 4H, H-3), 1.77 (m, 2H, H-2′′), 1.41 (m, 2H, H-3′′), 1.41 (m, 2H, H-4′′), 1.41 (m, 2H, H-5′′), 0.94 (t, 3H, H-6′′, 6.6); 13C-NMR (150 MHz, CD3OD) δ 199.81 (COPh), 135.06 (C-1′), 134.59 (C-4′), 130.02 (C-2′), 129.87 (C-3′), 79.34 (C-1), 45.63 (C-1′′), 36.31 (C-2), 32.48 (C-2′′), 27.99 (C-3′′), 27.31 (C-4′′), 26.70 (C-3), 23.50 (C-5′′), 14.30 (C-6′′); MS(CI+): 274 (MH+); 168 (M-COPh)+. HRMS: m/z calc. for C18H27NO (MH+) 274.2170; found 274.2184.
Synthesis of [6c-6′c]: n-Propylamine was used as amine component in the reaction. The mixture was purified by preparative HPLC using method D to give the desired products.
2-Phenyl-2-(propylamino)cyclohexanone (6c): Yellowish oil (27% yield); HPLC analysis Rt = 21.3 min (analytical method A); 1H-NMR (300 MHz, CD3OD) δ 7.46–7.62 (m, 2H, H-2′), 7.46–7.62 (m, 2H, H-3′), 7.46–7.62 (m, 1H, H-4′), 3.27 (dd, 1H, Ha-3, 13.8, 5.7, 3), 2.68 (m, 1H, Ha-1′′), 2.34–2.51 (m, 2H, H-6), 2.34–2.51 (m, 1H, Hb-1′′), 1.95–2.19 (m, 1H, Hb-3), 1.95–2.19 (m, 1H, Ha-4), 1.95–2.19 (m, 1H, Ha-5), 1.56–1.82 (m, 1H, Hb-4), 1.56–1.82 (m, 1H, Hb-5), 1.56–1.82 (m, 2H, H-2′′), 0.89 (t, 3H, H-3′′, 7.5); 13C-NMR (75 MHz, CD3OD) δ 207.03 (C-1), 131.79 (C-4′), 131.44 (C-1′), 131.16 (C-3′), 129.59 (C-2′), 73.44 (C-2), 44.95 (C-1′′), 40.23 (C-6), 33.57 (C-3), 28.61 (C-5), 22.75 (C-4), 20.73 (C-2′′), 11.24 (C-3′′); MS(CI+): 232 (MH+); 203 (M-Et)+; 174 (M-NHPro)+. HRMS: m/z calc. for C15H21NO (MH+) 232.1701; found 232.1697.
Phenyl(1-(propylamino)cyclopentyl)methanone (6'c): Yellowish oil (16% yield); HPLC analysis Rt = 21.5 min (analytical method A); 1H-NMR (300 MHz, CD3OD) δ 7.86 (m, 2H, H-2′), 7.66 (m, 1H, H-4′), 7.55 (m, 2H, H-3′), 2.94 (m, 2H, H-1′′), 2.62 (m, 2H, Ha-2), 2.17 (m, 2H, Hb-2), 2.17 (m, 4H, H-3), 1.82 (m, 2H, H-2′′), 1.06 (t, 3H, H-3′′, 7.5); 13C-NMR (75 MHz, CD3OD) δ 199.82 (COPh), 135.06 (C-1′), 134.59 (C-4′), 130.03 (C-2′), 129.88 (C-3′), 79.31 (C-1), 47.12 (C-1′′), 36.29 (C-2), 26.71 (C-3), 21.47 (C-2′′), 11.34 (C-3′′); MS(CI+): 232 (MH+); 126 (M-COPh)+. HRMS: m/z calc. for C15H21NO (MH+) 232.1701; found 232.1709.
Synthesis of [6d-6′d]: i-Propylamine was used as amine component in the reaction. The mixture was purified by preparative HPLC using method D to give the desired products.
2-(Isopropylamino)-2-phenylcyclohexanone (6d): White solid (56% yield); HPLC analysis Rt = 20.0 min (analytical method A); 1H-NMR (300 MHz, CD3OD) δ 7.50–7.63 (m, 2H, H-2′), 7.50–7.63 (m, 2H, H-3′), 7.50–7.63 (m, 1H, H-4′), 3.39 (sep, 1H, Ha-1′′, 6.6), 2.48 (m, 2H, H-6), 2.23 (ddd, 1H, Ha-3, 12.6, 5.7, 2.7), 2.04–2.16 (m, 1H, Hb-3), 2.04–2.16 (m, 1H, Ha-5), 1.94–1.97 (m, 1H, Ha-4), 1.72–1.86 (m, 1H, Hb-4), 1.72–1.86 (m, 1H, Hb-5), 0.95 (d, 6H, H-2′′, 6.6); 13C-NMR (75 MHz, CD3OD) δ 207.00 (C-1), 132.00 (C-4′), 131.95 (C-1′), 131.35 (C-3′), 129.87 (C-2′), 74.25 (C-2), 44.58 (C-1′′), 40.23 (C-6), 34.54 (C-3), 28.74 (C-5), 22.78 (C-4), 21.48** (C-2′′), 21.46** (C-2′′); MS(CI+): 232 (MH+); 174 (M−NHiPr)+. HRMS: m/z calc.calc. for C15H21NO (MH+) 232.1701; found 232.1649.
(1-(Isopropylamino)cyclopentyl)(phenyl)methanone (6'd): Yellowish oil (24% yield); HPLC analysis Rt = 21.0 min (analytical method A); 1H-NMR (300 MHz, CD3OD) δ 7.85 (m, 2H, H-2′), 7.67 (m, 1H, H-4′), 7.55 (m, 2H, H-3′), 3.44 (sep, 1H, H-1′′, 6.3), 2.64 (m, 2H, Ha-2), 2.27 (m, 2H, Hb-2), 2.06 (m, 4H, H-3), 1.41 (d, 6H, H-2′′, 6.3); 13C-NMR (75 MHz, CD3OD) δ 200.13 (COPh), 135.25 (C-1′), 134.52 (C-4′), 130.05 (C-2′), 129.87 (C-3′), 80.08 (C-1), 51.87 (C-1′′), 36.63 (C-2), 26.22 (C-3), 21.87 (C-2′′); MS(CI+): 232 (MH+); 126 (M−COPh)+. HRMS: m/z calc. for C15H21NO (MH+) 232.1701; found 232.1646.
Synthesis of [6e-6′e]: n-Butylamine was used as amine component in the reaction. The mixture was purified by preparative HPLC using method D to give the desired products.
2-(Butylamino)-2-phenylcyclohexanone (6e): Yellowish solid (58% yield); HPLC analysis Rt = 22.7 min (analytical method A); 1H-NMR (600 MHz, CD3OD) δ 7.58 (m, 2H, H-3′), 7.58 (m, 1H, H-4′), 7.48 (m, 2H, H-2′), 3.27 (ddd, 1H, Ha-3, 13.8, 6.3, 3), 2.72 (ddd, 1H, Ha-1′′, 12, 10.2, 6), 2.40–2.52 (m, 2H, H-6), 2.40–2.52 (m, 1H, Hb-1′′), 2.04–2.10 (m, 1H, Hb-3), 2.04–2.10 (m, 1H, Ha-5), 1.97 (m, 1H, Ha-4), 1.78 (m, 1H, Hb-4), 1.78 (m, 1H, Hb-5), 1.58 (m, 2H, H-2′′), 1.30 (m, 2H, H-3′′), 0.88 (t, 3H, H-4′′, 7.2); 13C-NMR (75 MHz, CD3OD) δ 207.08 (C-1), 132.50 (C-1′), 131.85 (C-4′), 131.20 (C-3′), 129.65 (C-2′), 73.52 (C-2), 43.19 (C-1′′), 40.26 (C-6), 33.65 (C-3), 29.32 (C-2′′), 28.67 (C-5), 22.78 (C-4), 20.89 (C-3′′), 13.83 (C-4′′); MS(CI+): 246 (MH+); 217 (M-Et)+; 174 (M−NHBu)+. HRMS: m/z calc. for C16H23NO (MH+) 246.1857; found 246.1828.
(1-(Butylamino)cyclopentyl)(phenyl)methanone (6'e): Yellowish oil (14% yield); HPLC analysis Rt = 23.9 min (analytical method A); 1H-NMR (600 MHz, CDCl3) δ 7.87 (m, 2H, H-2′), 7.66 (m, 1H, H-4′), 7.55 (m, 2H, H-3′), 2.97 (m, 2H, H-1′′), 2.65 (m, 2H, Ha-2), 2.24 (m, 2H, Hb-2), 2.11 (m, 4H, H-3), 1.78 (m, 2H, H-2′′), 1.48 (m, 2H, H-3′′), 1.01 (t, 3H, H-4′′, 7.2); 13C-NMR (75 MHz, CDCl3) δ 199.79 (COPh), 135.05 (C-1′), 134.52 (C-4′), 129.84 (C-2′), 129.78 (C-3′), 79.33 (C-1), 45.40 (C-1′′), 36.28 (C-2), 30.01 (C-2′′), 26.70 (C-3), 20.87 (C-3′′), 13.95 (C-4′′); MS(CI+): 246 (MH+); 140 (M−COPh)+. HRMS: m/z calc. for C16H23NO (MH+) 246.1857; found 246.1818.
Synthesis of [6f-6′f]: Aniline was used as amine component in the reaction. The mixture was purified by preparative HPLC using method F to give the desired products.
2-Phenyl-2-(phenylamino)cyclohexanone (6f): White solid (8% yield); HPLC analysis Rt = 24.3 min (analytical method A); 1H-NMR (600 MHz, CDCl3) δ 9.46 (bs, 1H##, NH), 7.37 (m, 2H, H-3′), 7.37 (m, 1H, H-4′), 7.18 (m, 2H, H-2′), 7.10 (m, 2H, H-3′′), 7.10 (m, 1H, H-4′′), 6.77 (m, 2H, H-2′′), 2.94 (dq, 1He#, Ha-3, 14, 3.5), 2.51 (dddd, 1He#, Ha-6, 14, 4, 3.5, 2), 2.42 (ddd, 1Ha#, Hb-3, 14, 12.5, 4), 2.35 (ddd, 1Ha#, Hb-6, 14, 12.5, 6) 2.09 (ddqd, 1He#, Ha-5, 13, 6, 4, 3.5), 1.93 (dqdd, 1He#, Ha-4, 13, 4, 3.5, 2), 1.84 (dtdd, 1Ha#, Hb-5, 13, 12.5, 4, 3.5), 1.75 (dtdd, 1Ha#, Hb-4, 13, 12.5, 4, 3.5); 13C-NMR (75 MHz, CDCl3) δ 206.54 (C-1), 134.50 (C-1′′), 132.20 (C-1′), 130.03 (C-4′), 129.54 (C-3′), 129.01 (C-3′′), 128.46 (C-2′), 126.66 (C-4′′), 123.29 (C-2′′), 73.86 (C-2), 39.22 (C-6), 34.03 (C-3), 27.39 (C-5), 22.01 (C-4); MS(CI+): 266 (MH+); 265 (M+•); 237 (M−Et)+; 93 (NC6H7). HRMS: m/z calc.calc. for C18H19NO (M+) 265.1467; found 265.1494.
Phenyl(1-(phenylamino)cyclopentyl)methanone (6'f): Yellow-green solid (8% yield); HPLC analysis Rt = 31.6 min (analytical method A); 1H-NMR (300 MHz, CDCl3) δ 8.07 (m, 2H, H-2′), 7.46 (m, 1H, H-4′), 7.35 (m, 2H, H-3′), 7.11 (m, 2H, H-3′′), 6.80 (m, 1H, H-4′′), 6.80 (bs, 1H##, NH), 6.66 (m, 2H, H-2′′), 2.54 (m, 2H, Ha-2), 2.09 (m, 2H, Hb-2), 1.84 (m, 4H, H-3); 13C-NMR (75 MHz, CDCl3) δ 203.72 (COPh), 143.07 (C-1′′), 135.57 (C-1′), 132.68 (C-4′), 129.45 (C-2′), 129.15 (C-3′′), 128.33 (C-3′), 120.60 (C-4′′), 116.41 (C-2′′), 74.92 (C-1), 37.47 (C-2), 25.08 (C-3); MS(CI+): 266 (MH+); 265 (M+•); 160 (M−COPh)+. HRMS: m/z calc. for C18H19NO (M+•) 265.1467; found 265.1500.
Synthesis of [6g-6′g]: 4-Chloroaniline was used as amine component in the reaction. The mixture was purified by preparative HPLC using method F to give the desired products.
2-(4-Chlorophenyl)-2-(phenylamino)cyclohexanone (6g): Yellow-brown solid (12% yield); HPLC analysis Rt = 32.2 min (analytical method A); 1H-NMR (300 MHz, CDCl3) δ 7.58 (bs, 1H##, NH), 7.36 (m, 2H, H-3′), 7.36 (m, 1H, H-4′), 7.25 (m, 2H, H-2′), 7.02 (m, 2H, H-2′′), 6.62 (m, 1H, H-3′′), 3.00 (m, 1H, Ha-3), 2.49 (m, 1H, Ha-6), 2.31 (m, 1H, Hb-3), 2.31 (m, 1H, Hb-6) 1.91 (m, 2H, H-4), 1.91 (m, 2H, H-5); 13C-NMR (75 MHz, CDCl3) δ 206.81 (C-1), 136.24 (C-1′′), 132.99 (C-1′), 129.46 (C-4′), 129.41* (C-3′), 128.94* (C-3′′), 128.38 (C-4′′), 128.30* (C-2′), 122.24 (C-2′′), 72.30 (C-2), 39.09 (C-6), 35.51 (C-3), 27.73 (C-5), 22.32 (C-4); MS(ES+): 322 (M+Na)+; 300 (MH+); 174 (M−NHC6H4Cl)+. HRMS: m/z calc. for C18H18NOCl (MH+) 300.1155; found 300.1135.
(4-Chlorophenyl)(1-(phenylamino)cyclopentyl)methanone (6'g): Yellow-brown solid (32% yield); HPLC analysis Rt = 34.0 min (analytical method A); 1H-NMR (300 MHz, CDCl3) δ 8.04 (m, 2H, H-2′), 7.44 (m, 1H, H-4′), 7.33 (m, 2H, H-3′), 6.98 (m, 2H, H-3′′), 6.43 (m, 2H, H-2′′), 4.75 (bs, 1H##, NH), 2.55 (m, 2H, Ha-2), 2.00 (m, 2H, Hb-2), 1.81 (m, 4H, H-3); 13C-NMR (75 MHz, CDCl3) δ 204.43 (COPh), 144.25 (C-1′′), 135.89 (C-1′), 132.34 (C-4′), 128.96* (C-2′), 128.96* (C-3′′), 128.06* (C-3′), 122.51 (C-4′′), 114.89 (C-2′′), 72.82 (C-1), 37.77 (C-2), 24.85 (C-3); MS(CI+): 300 (MH+); 194 (M−COPh)+. HRMS: m/z calc.calc. for C18H18NOCl (MH+) 300.1155; found 300.1164.
3.5. General Procedure for the Parallel Synthesis of Library Compounds 7a–g, 7′a–g
To seven pressure-tubes each containing 2-hydroxy-2-methylcyclohexanone (3′, 128 mg, 1 mmol) were added the appropriate primary amine (benzylamine, hexylamine, propylamine, isopropylamine, butylamine, aniline, 4-chloroaniline, 1.15 mmol). The tubes were flushed with argon, sealed and heated at 200 °C overnight in a Radley’s combinatorial station with stirring. The aliphatic residues were concentrated and purified by silica gel column chromatography (100% CH2Cl2, CH2Cl2–MeOH 15:0.1 and then CH2Cl2–MeOH 15:1) to give the desired products. All the cleaned products were treated with 0.1M HCl and washed with ether to give their corresponding hydrochloride salts.
The aromatic residues were diluted with ether and extracted with 0.1 M HCl. The acidic phase washed with ether several times and the combined ether phases were dried over MgSO4 and concentrated. Preparative HPLC were performed with method D, according to the elution times obtained by analytical HPLC using method A.
3.6. Characterization Data for Compounds 7a–g, 7′a–g
For the NMR data numbering system of compounds
7a–g,
7′a–g please refer to
Table 6.
Table 6.
Numbering system for NMR chemical shifts attribution for library 7a–g, 7′a–g.
Table 6.
Numbering system for NMR chemical shifts attribution for library 7a–g, 7′a–g.
![Molecules 17 06784 i003]() |
Synthesis of [7a-7′a]: Benzylamine was used as amine component in the reaction. The mixture was purified by preparative HPLC using method D to give the desired products as mixtures.
2-(Benzylamino)-2-methylcyclohexanone + 1-(1-(benzylamino)cyclopentyl)ethanone (7a+7′a). Mixture of products 1:1 as white solid (48% yield); HPLC analysis Rt = 3.4 min (analytical method B); 1H-NMR (600 MHz, CD3OD) 7a: δ 7.46–7.56 (m, 2H, H-2′), 7.46–7.56 (m, 2H, H-3′), 7.46–7.56 (m, 1H, H-4′), 4.20 (d, 1H, CH2-Bn, 12.6), 4.10 (d, 1H, CH2-Bn, 12.6), 2.82 (td, 1H, Ha-6, 14.4, 6.6), 2.45 (ddt, 1H, Hb-6, 14.4, 4.2, 1.8), 2.28 (ddd, 1H, Ha-3, 12, 6, 3.6), 2.18 (m, 1H, Ha-5), 2.11 (m, 1H, Ha-4), 2.00 (m, 1H, Hb-3), 1.95 (m, 1H, Hb-4), 1.71 (s, 3H, Me), 1.68(m, 1H, Hb-5). 6′a: δ 7.47–7.56 (m, 2H, H-2′), 7.47–7.56 (m, 2H, H-3′), 7.47–7.56 (m, 1H, H-4′), 4.07 (s, 2H, CH2-Bn), 2.35 (m, 2H, Ha-2), 2.34 (s, 3H, Me), 2.11(m, 2H, Hb-2), 2.02 (m, 4H, H-3); 13C-NMR (150 MHz, CD3OD) 7a: δ 208.34 (C-1), 132.99* (C-1′), 131.16* (C-3′), 130.71* (C-4′), 130.27* (C-2′), 69.13 (C-2), 47.29 (CH2-Bn), 38.98 (C-6), 36.59 (C-3), 28.85 (C-5), 22.46 (C-4), 19.68 (Me). 7′a: δ 205.79 (COMe), 131.26* (C-3′), 130.71* (C-4′), 130.32* (C-2′), 130.18* (C-1′), 78.46 (C-1), 49.32 (CH2-Bn), 35.00 (C-2), 27.23 (C-3), 24.45 (Me); MS(CI+): 218 (MH+); 174 (M−COMe)+; 112 (M−NHC7H7+). HRMS: m/z calc. for C14H19NO (MH+) 218.1545; found 218.1561.
Synthesis of [7b-7′b]: n-Hexylamine was used as amine component in the reaction. The residue was purified by silica gel column chromatography (100% CH2Cl2, CH2Cl2–MeOH 15:0.1 and then CH2Cl2–MeOH 15:1, ninhydrine) to give the desired product.
2-(Hexylamino)-2-methylcyclohexanone (7b): Yellow-brown oil (28% yield); 1H-NMR (300 MHz, CD3OD) δ 2.93 (m, 2H, H-1′), 2.80 (td, 1H, Ha-6, 14.1, 6.3), 2.42 (m, 1H, Hb-6), 2.18 (m, 2H, H-3), 1.94 (m, 1H, Ha-4), 1.94 (m, 1H, Ha-5), 1.90 (m, 1H, Hb-4), 1.90 (m, 1H, Hb-5), 1.68 (m, 2H, H-2’), 1.62 (s, 3H, Me), 1.38 (m, 2H, H-3′), 1.38 (m, 2H, H-4′), 1.38 (m, 2H, H-5′), 0.93 (t, 3H, H-6′); 13C-NMR (75 MHz, CD3OD) δ 208.53 (C-1), 68.39 (C-2), 43.04 (C-1′), 38.84 (C-6), 36.31 (C-3), 32.44 (C-2′), 27.76 (C-5), 27.76 (C-3′), 27.34 (C-4′), 23.47 (C-5′), 22.14 (C-4), 19.45 (Me), 14.27 (C-6′); MS(CI+): 280 (MH+); MS(CI+): 212 (MH+); 183 (M−Et)+; 154 (M-Bu)+; 112 (M−NHHex)+. HRMS: m/z calc. for C13H25NO (MH+) 212.2014; found 212.1973.
1-(1-(Hexylamino)cyclopentyl)ethanone (7′b): Yellow oil (8% yield); 1H-NMR (300 MHz, CD3OD) δ 2.87 (m, 2H, H-1′), 2.29 (s, 3H, Me), 2.28 (m, 2H, Ha-2), 2.02 (m, 2H, Hb-2), 2.02 (m, 4H, H-3), 1.74 (m, 2H, H-2′), 1.37 (m, 2H, H-3′), 1.37 (m, 2H, H-4′), 1.37 (m, 2H, H-5′), 0.93 (t, 3H, H-6′, 6.6); 13C-NMR (150 MHz, CD3OD) δ 205.80 (COMe), 77.99 (C-1), 45.38 (C-1′), 34.75 (C-2), 32.40 (C-2′), 27.89 (C-3′), 27.25 (C-4′), 27.14 (C-3), 24.36 (Me), 23.45 (C-5′), 14.26 (C-6′); MS(CI+): 212 (MH+); 168 (M−COMe)+. HRMS: m/z calc. for C13H25NO (MH+) 212.2014; found 212.1979.
Synthesis of [7c-7′c]: n-propylamine was used as amine component in the reaction. The residue was purified by silica gel column chromatography (100% CH2Cl2, CH2Cl2–MeOH 15:0.1 and then CH2Cl2–MeOH 15:1) to give the desired products.
2-Methyl-2-(propylamino)cyclohexanone (7c): Yellow-brown oil (16% yield); 1H-NMR (600 MHz, CD3OD) δ 2.95 (m, 1H, Ha-1′), 2.85 (m, 1H, Hb-1′), 2.82 (td, 1H, Ha-6, 14.4, 6.6), 2.42 (m, 1H, Hb-6), 2.25 (m, 1H, Ha-3), 2.15 (m, 1H, Ha-5), 1.95 (m, 2H, H-4), 1.92 (m, 1H, Hb-3), 1.73 (m, 2H, H-2′), 1.68 (m, 1H, Hb-5), 1.63 (s, 3H, Me), 1.04 (t, 3H, H-3′, 7.8); 13C-NMR (150 MHz, CD3OD) δ 208.51 (C-1), 68.37 (C-2), 45.54 (C-1′), 38.86 (C-6), 36.31 (C-3), 27.77 (C-5), 22.27 (C-4), 21.22 (C-2′), 19.48 (Me), 11.34 (C-3′); MS(CI+): 280 (MH+); MS(CI+): 170 (MH+); 112 (M−NHPro)+. HRMS: m/z calc. for C10H19NO (MH+) 170.1545; found 170.1550.
1-(1-(Propylamino)cyclopentyl)ethanone (7'c): Yellow-brown oil (20% yield); 1H-NMR (300 MHz, CD3OD) δ 2.85 (m, 2H, H-1′), 2.30 (m, 2H, Ha-2), 2.29 (s, 3H, Me), 2.01 (m, 2H, Hb-2), 2.01 (m, 4H, H-3), 1.76 (m, 2H, H-2′), 1.03 (t, 3H, H-3′, 7.5); 13C-NMR (75 MHz, CD3OD) δ 205.78 (COMe), 77.93 (C-1), 46.96 (C-1′), 34.74 (C-2), 27.18 (C-3), 24.46 (Me), 21.35 (C-2′), 11.32 (C-3′); MS(CI+): 170 (MH+); 126 (M−COMe)+. HRMS: m/z calc. for C10H19NO (MH+) 170.1545; found 170.1527.
Synthesis of [7d-7′d]: iso-propylamine was used as amine component in the reaction. The residue was purified by silica gel column chromatography (100% CH2Cl2, CH2Cl2–MeOH 15:0.1 and then CH2Cl2–MeOH 15:1) to give product [7d].
2-(Isopropylamino)-2-methylcyclohexanone (7d). Yellow oil (18% yield); 1H-NMR (300 MHz, CD3OD) δ 3.50 (sep, 1H, Ha-1′, 6.6), 2.79 (ddd, 1H, Ha-6, 14.7, 14.1, 6.6), 2.44 (m, 1H, Hb-6), 2.23 (ddd, 1H, Ha-3, 12, 5.7, 3), 1.90–2.18 (m, 1H, Hb-3), 1.90–2.18 (m, 1H, Ha-4), 1.90–2.18 (m, 1H, Hb-4), 1.90–2.18 (m, 1H, Ha-5), 1.90–2.18 (m, 1H, Hb-5), 1.66 (s, 3H, Me), 1.39 (d, 3H, Ha-2′, 6.6), 1.39 (d, 3H, Hb-2′, 6.6); 13C-NMR (75 MHz, CD3OD) δ 208.25 (C-1), 70.00 (C-2), 49.77 (C-1′), 38.73 (C-6), 36.63 (C-3), 27.49 (C-5), 22.59 (C-4), 22.08 (Me), 22.08 (C-2′), 20.82 (C-2′); MS(CI+): 170 (MH+); 141 (M−Et)+; 126 (M−iPr)+; 112 (M−NHiPr)+. HRMS: m/z calc. for C10H19NO (MH+) 170.1545; found 170.1640.
Synthesis of [7e-7′e]: n-butylamine was used as amine component in the reaction. The residue was purified by silica gel column chromatography (100% CH2Cl2, CH2Cl2–MeOH 15:0.1 and then CH2Cl2–MeOH 15:1) to give the desired products.
2-(Butylamino)-2-methylcyclohexanone (7e). Yellowish oil (16% yield); 1H-NMR (300 MHz, CD3OD) δ 2.96 (m, 2H, H-1′, 6.6), 2.80 (td, 1H, Ha-6, 14.4, 6.3), 2.42 (m, 1H, Hb-6), 2.21 (m, 2H, H-3), 1.94 (m, 1H, Ha-4), 1.94 (m, 1H, Ha-5), 1.72 (m, 1H, Hb-4), 1.72 (m, 1H, Hb-5), 1.72 (m, 1H, Ha-2′), 1.63 (s, 3H, Me), 1.48 (m, 1H, Hb-2′), 1.48 (m, 2H, H-3′), 1.00 (t, 3H, H-4′, 7.5); 13C-NMR (75 MHz, CD3OD) δ 208.53 (C-1), 68.41 (C-2), 42.84 (C-1′), 38.86 (C-6), 36.03 (C-3), 29.77 (C-2′), 27.76 (C-5), 22.15 (C-4), 20.91 (C-3′), 19.50 (Me), 13.95 (C-4′); MS(CI+): 184 (MH+); 140 (M−Pro)+; 126 (M−Bu)+; 112 (M−NHBu)+. HRMS: m/z calc. for C11H21NO (MH+) 184.1701; found 184.1712.
1-(1-(Butylamino)cyclopentyl)ethanone (7'e). Yellowish oil (34% yield); 1H-NMR (300 MHz, CD3OD) δ 2.88 (m, 2H, H-1′), 2.30 (s, 3H, Me), 2.28 (m, 2H, Ha-2), 2.01 (m, 2H, Hb-2), 2.01 (m, 4H, H-3), 1.71 (m, 2H, H-2′), 1.44 (m, 2H, H-3′), 0.98 (t, 3H, H-4′, 7.5); 13C-NMR (75 MHz, CD3OD) δ 205.83 (COMe), 77.97 (C-1), 45.21 (C-1′), 34.76 (C-2), 29.90 (C-2′), 27.17 (C-3), 24.49 (Me), 20.83 (C-3′), 13.93 (C-4′); MS(CI+): 184 (MH+); 140 (M−COMe)+. HRMS: m/z calc. for C10H19NO (MH+) 184.1701; found 184.1738.
Synthesis of [7f-7′f]: aniline was used as amine component in the reaction. The organic phase was purified by preparative reverse-HPLC (method D) to give the product [7'f].
1-(1-(Phenylamino)cyclopentyl)ethanone (7'f). Yellow oil (10% yield); HPLC analysis Rt = 4.9 min (analytical method B); 1H-NMR (300 MHz, CDCl3) δ 7.20 (m, 2H, H-3′), 6.85 (m, 1H, H-4′), 6.65 (m, 2H, H-2′), 4.36 (bs, 1H, NH), 2.23 (m, 2H, Ha-2), 2.23 (s, 3H, Me), 1.81 (m, 2H, Hb-2), 1.81 (m, 4H, H-3); 13C-NMR (75 MHz, CDCl3) δ 211.39 (COMe), 142.58 (C-1′), 129.47 (C-3′), 120.68 (C-4′), 116.18 (C-2′), 74.69 (C-1), 35.67 (C-2), 25.27 (Me), 25.08 (C-3); MS(CI+): 204 (MH+); 203 (M+•); 160 (M−COMe)+. HRMS: m/z calc. for C13H17NO (M+•) 203.1310; found 203.1319.
Synthesis of [7g-7′g]: 4-Cl-aniline was used as amine component in the reaction. The organic phase was purified by preparative reverse-HPLC (method D) to give the product [7'g].
1-(1-((3-Chlorophenyl)amino)cyclopentyl)ethanone (7'g). Yellow oil (21% yield); HPLC analysis Rt = 5.8 min (analytical method B); 1H-NMR (300 MHz, CDCl3) δ 7.48 (bs, 1H, NH), 7.18 (m, 2H, H-2′), 6.70 (m, 2H, H-3′), 2.22 (s, 3H, Me), 2.20 (m, 2H, Ha-2), 1.90 (m, 2H, Hb-2), 1.77 (m, 4H, H-3); 13C-NMR (75 MHz, CDCl3) δ 219.59 (COMe), 139.78 (C-1′), 129.49 (C-3′), 127.08 (C-4′), 118.50 (C-2′), 75.55 (C-1), 35.34 (C-2), 25.20 (C-3), 24.98 (Me); MS (CI+): 238 (MH+); 194 (M−COMe)+. HRMS: m/z calc. for C13H16NO (MH+) 238.0999; found 238.0957.
3.7. Microwave-Assisted Syntheses
Synthesis of [6a-6′a]+ compound 8: To a microwave flask containing 95 mg (0.5 mmol) of 2-hydroxy-2-phenylcyclohexanone 3, was added 0.575 mmol of benzylamine and NMP (1.5 mL). The flask was flushed with argon and sealed. The reaction mixture was heated at 230 °C for 10 min (until starting reagents disappeared according to analytical HPLC method B). The residue was diluted with ten folds of water and TFA was added until clearness. The obtained solution was filtered and purified by preparative reverse-HPLC (method E).
2-(Benzylamino)-2-phenylcyclohexanone (6a). White solid (30% yield); HPLC analysis Rt = 4.0 min (analytical method B); 1H-NMR (300 MHz, CD3OD) δ 7.54–7.67 (m, 2H, H-2′), 7.54–7.67 (m, 2H, H-3′), 7.54–7.67 (m, 1H, H-4′), 7.46–7.56 (m, 2H, H-3′′), 7.46–7.56 (m, 1H, H-4′′), 7.31–7.42 (m, 2H, H-2′′), 3.78 (d, 1H, CH2-Bn, 12.9), 3.74 (d, 1H, CH2-Bn, 12.9), 3.33 (ddd, 1H, Ha-3, 13.5, 5.7, 2.7), 2.54 (m, 2H, H-6), 2.08–2.22 (m, 1H, Hb-3), 2.08–2.22 (m, 1H, Ha-5), 1.96–2.00 (m, 1H, Ha-4), 1.73–1.88 (m, 1H, Hb-4), 1.73–1.88 (m, 1H, Hb-5); 13C-NMR (75 MHz, CD3OD) δ 206.96 (C-1), 132.43* (C-1′′), 131.98* (C-4′), 131.51* (C-1′), 131.29* (C-3′), 131.17* (C-3′′), 130.59* (C-4′′), 130.14* (C-2′′), 129.90* (C-2′), 74.28 (C-2), 47.90 (CH2-Bn), 40.47 (C-6), 34.38 (C-3), 28.74 (C-5), 22.80 (C-4); MS(CI+): 280 (MH+); 91 (C7H7+). HRMS: m/z calc. for C19H21NO (MH+) 280.1701; found 280.1694.
(1-(Benzylamino)cyclopentyl)(phenyl)methanone (6'a). White solid (60% yield); HPLC analysis Rt = 4.2 min (analytical method B); 1H-NMR (300 MHz, CDCl3) δ 8.23 (m, 2H, H-2′), 7.43 (m, 2H, H-3′), 7.52 (m, 1H, H-4′), 7.21 (m, 2H, H-3′′), 7.19 (m, 2H, H-4′′), 7.05 (m, 2H, H-2′′), 3.49 (s, 2H, CH2-Bn), 2.40 (m, 2H, Ha-2), 1.90 (m, 2H, Hb-2), 1.80 (m, 2H, Ha-3), 1.69 (m, 2H, Hb-3); 13C-NMR (75 MHz, CDCl3) δ 204.30 (COPh), 140.24 (C-1′′), 136.28 (C-1′), 132.17 (C-4′), 129.33 (C-2′), 128.30 (C-3′′), 128.22 (C-2′′), 128.10 (C-3′), 126.99 (C-4′′), 75.17 (C-1), 49.32 (CH2-Bn), 36.68 (C-2), 24.66 (C-3); MS(CI+): 280 (MH+); 174 (M-COPh)+. HRMS: m/z calc. for C19H21NO (MH+) 280.1701; found 280.1720.
5-Benzyl-[1,1'-biphenyl]-2-amine (8). (8% yield). HPLC analysis Rt = 4.8 min (analytical method B); 1H-NMR (600 MHz, CDCl3) δ 7.41–7.43 (m, 4H, H-2', H-3'), 7.32 (m, 1H, H-4'), 7.27 (m, 2H, H-3''), 7.21 (m, 2H, H-2"), 7.17 (m, 1H, H-4"), 6.98 (m, 1H, H-3), 6.97 (m, 1H, H-5), 6.72 (m, 1H, H-6), 3.91 (s, 2H, CH2-Bn), 3.60 (m, 2H, NH2); 13C-NMR (75 MHz, CDCl3) δ 144.75 (C-1"), 144.28 (C-1), 139.46 (C-1'), 131.55 (C-4), 130.90 (C-3), 129.09 (C-3'), 128.93 (C-5), 128.81* (C-2"), 128.75* (C-2'), 128.39 (C-3'), 127.91 (C-2), 127.13 (C-4'), 125.88 (C-4"), 116.02 (C-6), 41.02 (CH2-Bn); MS(CI+): 260 (MH+); 259 (M+). HRMS: m/z calc. for C19H17N (M+) 259.1361; found 259.1368.
Synthesis of [6b-6′b]: To a microwave flask containing 95 mg (0.5 mmol) 2-hydroxy-2-phenylcyclohexanone 3, was added 0.575 mmol of hexylamine and NMP (1.5 mL). The flask was flushed with argon and sealed. The reaction mixture was heated at 230 °C for 20 min (until starting reagents disappeared according to analytical HPLC method B). The residue was diluted with ten folds of water and TFA was added to clearness. The resulting solution was filtered and purified by preparative reverse-HPLC (method E).
2-(Hexylamino)-2-phenylcyclohexanone (6b). Yellowish oil (20% yield); HPLC analysis Rt = 4.5 min (analytical method B); 1H-NMR (300 MHz, CD3OD) δ 7.49–7.61 (m, 2H, H-2′), 7.49–7.61 (m, 2H, H-3′), 7.49–7.61 (m, 1H, H-4′), 3.29 (m, 2H, Ha-3), 2.72 (m, 1H, Ha-1′′), 2.47 (m, 1H, Hb-1′′), 2.47 (m, 2H, H-6), 1.96–2.16 (m, 1H, Hb-3), 1.96–2.16 (m, 1H, Ha-4), 1.96–2.16 (m, 1H, Ha-5), 1.82 (m, 1H, Hb-4), 1.82 (m, 1H, Hb-5), 1.59 (m, 2H, H-2′′), 1.28 (m, 2H, H-3′′), 1.28 (m, 2H, H-4′′), 1.28 (m, 2H, H-5′′), 0.88 (t, 3H, H-6′′, 6.6); 13C-NMR (75 MHz, CD3OD) δ 207.00 (C-1), 131.77 (C-4′), 131.49 (C-1′), 131.14 (C-3′), 129.59 (C-2′), 73.48 (C-2), 43.47 (C-1′′), 40.24 (C-6), 33.61 (C-3), 32.27 (C-2′′), 28.60 (C-5), 27.24 (C-3′′), 27.20 (C-4′′), 23.32 (C-5′′), 22.74 (C-4), 14.20 (C-6′′); HRMS: m/z calc. for C18H27NO (MH+) 274.2170; found 274.2173.
(1-(Hexylamino)cyclopentyl)(phenyl)methanone (6'b). Yellowish oil (10% yield); HPLC analysis Rt = 4.7 min (analytical method B); 1H-NMR (300 MHz, CD3OD) δ 7.86 (m, 2H, H-2′), 7.66 (m, 1H, H-4′), 7.55 (m, 2H, H-3′), 2.96 (m, 2H, H-1′′), 2.61 (m, 2H, Ha-2), 2.15 (m, 2H, Hb-2), 2.15 (m, 4H, H-3), 1.77 (m, 2H, H-2′′), 1.41 (m, 2H, H-3′′), 1.41 (m, 2H, H-4′′), 1.41 (m, 2H, H-5′′), 0.94 (t, 3H, H-6′′, 6.6); 13C-NMR (75 MHz, CD3OD) δ 199.81 (COPh), 135.06 (C-1′), 134.59 (C-4′), 130.02 (C-2′), 129.87 (C-3′), 79.34 (C-1), 45.63 (C-1′′), 36.31 (C-2), 32.48 (C-2′′), 27.99 (C-3′′), 27.31 (C-4′′), 26.70 (C-3), 23.50 (C-5′′), 14.30 (C-6′′); HRMS: m/z calc. for C18H27NO (MH+) 274.2170; found 274.2190.
Synthesis of [6c-6′c]: To a microwave flask containing 95 mg (0.5 mmol) 2-hydroxy-2-phenylcyclohexanone 3, was added 0.575 mmol of propylamine and NMP (1.5 mL). The flask was flushed with argon and sealed. The reaction mixture was heated at 230 °C for 30 min (until starting reagents disappeared according to analytical HPLC method B). The residue was diluted with ten folds of water and TFA was added to clearness. The resulting solution was filtered and purified by preparative reverse-HPLC (method E).
2-Phenyl-2-(propylamino)cyclohexanone (6c). Yellow oil (20% yield); HPLC analysis Rt = 3.6 min (analytical method B); 1H-NMR (300 MHz, CD3OD) δ 7.46–7.62 (m, 2H, H-2′), 7.46–7.62 (m, 2H, H-3′), 7.46–7.62 (m, 1H, H-4′), 3.27 (ddd, 1H, Ha-3, 13.8, 5.7, 3), 2.68 (m, 1H, Ha-1′′), 2.34–2.51 (m, 2H, H-6), 2.34–2.51 (m, 1H, Hb-1′′), 1.95–2.19 (m, 1H, Hb-3), 1.95–2.19 (m, 1H, Ha-4), 1.95–2.19 (m, 1H, Ha-5), 1.56–1.82 (m, 1H, Hb-4), 1.56–1.82 (m, 1H, Hb-5), 1.56–1.82 (m, 2H, H-2′′), 0.89 (t, 3H, H-3′′, 7.5); 13C-NMR (150 MHz, CD3OD) δ 207.03 (C-1), 131.79 (C-4′), 131.44 (C-1′), 131.16 (C-3′), 129.59 (C-2′), 73.44 (C-2), 44.95 (C-1′′), 40.23 (C-6), 33.57 (C-3), 28.61 (C-5), 22.75 (C-4), 20.73 (C-2′′), 11.24 (C-3′′); MS(CI+): 232 (MH+); 203 (M-Et)+; 174 (M−NHPr)+. HRMS: m/z calc. for C15H21NO (MH+) 232.1701; found 232.1714.
Phenyl(1-(propylamino)cyclopentyl)methanone (6'c). Yellow oil (20% yield); HPLC analysis Rt = 3.7 min (analytical method B); 1H-NMR (300 MHz, CD3OD) δ 7.86 (m, 2H, H-2’), 7.66 (m, 1H, H-4′), 7.55 (m, 2H, H-3′), 2.94 (m, 2H, H-1′′), 2.62 (m, 2H, Ha-2), 2.17 (m, 2H, Hb-2), 2.17 (m, 4H, H-3), 1.82 (m, 2H, H-2′′), 1.06 (t, 3H, H-3′′, 7.5); 13C-NMR (150 MHz, CD3OD) δ 199.82 (COPh), 135.06 (C-1′), 134.59 (C-4′), 130.03 (C-2′), 129.88 (C-3′), 79.31 (C-1), 47.12 (C-1′′), 36.29 (C-2), 26.71 (C-3), 21.47 (C-2′′), 11.34 (C-3′′); MS(CI+): 232 (MH+); 126 (M−COPh)+. HRMS: m/z calc. for C15H21NO (MH+) 232.1701; found 232.1673.
Synthesis of [6f-6′f]: To a microwave flask containing 95 mg (0.5 mmol) 2-hydroxy-2-phenylcyclohexanone 3, was added 0.575 mmol of aniline and NMP (1.5 mL). The flask was flushed with argon and sealed. The reaction mixture was heated at 230 °C for 30 min (until starting reagents disappeared according to analytical HPLC method B). The residue was diluted with twenty fold of saturated NaHCO3 solution and extracted with EtOAc. The organic phase was concentrated, diluted with ether and extracted with 0.1 M HCl. The acidic phase was washed with ether and concentrated. The residue was purified by preparative reverse-HPLC (method E).
Physical properties of phenyl(1-(phenylamino)cyclopentyl)methanone (6'f). Yellow-brown solid (14% yield); HPLC analysis Rt = 5.4 min (analytical method B); 1H-NMR (600 MHz, CDCl3) δ 8.07 (m, 2H, H-2′), 7.46 (m, 1H, H-4′), 7.35 (m, 2H, H-3′), 7.11 (m, 2H, H-3′′), 6.80 (m, 1H, H-4′′), 6.80 (bs, 1H##, NH), 6.66 (m, 2H, H-2′′), 2.54 (m, 2H, Ha-2), 2.09 (m, 2H, Hb-2), 1.84 (m, 4H, H-3); 13C-NMR (150 MHz, CDCl3) δ 203.72 (COPh), 143.07 (C-1′′), 135.57 (C-1′), 132.68 (C-4′), 129.45 (C-2′), 129.15 (C-3′′), 128.33 (C-3′), 120.60 (C-4′′), 116.41 (C-2′′), 74.92 (C-1), 37.47 (C-2), 25.08 (C-3); MS(ES+): 266 (MH+). HRMS: m/z calc. for C18H19NO (M+•) 265.1467; found 265.1483.
3.8. Experimental Protocols for Bioassays
To examine the protective effect of the synthesized ketamine derivatives, we used a sepsis model and examined the inflammatory response of treated animals. Sepsis was induced in mice by intra-peritoneal (i.p.) inoculation of a lethal dose of
Escherichia coli (
E. coli). Inoculation of animals with live bacteria has been a common tool for studying sepsis mechanisms and represents a model relevant to clinical practice [
34,
35]. Introduction of bacteria into the peritoneum initiates a rapid response which primarily induces inflammatory and immune cell migration into the infected compartment [
35]. Previous data showed a beneficial effect of ketamine on animal survival after
E. coli-induced sepsis. This effect was attributed to the anti-inflammatory activity of ketamine, as evidenced by decreased IL-6 and TNF-α levels in serum and peritoneal lavage of treated animals [
12,
13]. To determine the anti-inflammatory effects of ketamine analogues, mice were injected subcutaneously with 10 mg/kg of ketamine or its analogues prior to sepsis induction. As a control, mice were injected with saline. In order to measure systemic and local cytokines levels mice were anesthetized at 16 h after sepsis induction. Blood was drawn by intracardiac puncture and a peritoneal lavage was performed. The concentrations of IL-6 and TNFα were used as inflammation markers and were measured by enzyme-linked immune-sorbent assay (ELISA) as previously described [
10]. The ELISA kits that were used are sandwich ELISA kits with 96 well strip plates. These kits are designed for the accurate quantitation of analytes from cell culture supernatant, serum, plasma or other body fluids. In addition, the behavior of mice was observed at time of ketamine analogues injection to confirm that there are no immediate side effects. The analogues-treated mice were also monitored prior to their anesthetization and compared to ketamine and saline treated mice.
3.8.1. Mice, Bacterial Strains, and Drugs
CD1 female mice aged 10 to 12 weeks (Harlan, Jerusalem, Israel) were maintained in the animal laboratory of the Soroka Medical Center. Experiments were done with the permission of the Ben-Gurion University of the Negev Committee for Ethical Care and Use of Animals in Experiments (Beer-Sheva, Israel). E. coli were grown in Luria-Bertani broth (Conda Laboratories, Madrid, Spain) and harvested during the log phase. Bacteria aliquots were stored frozen in LB broth containing 30% glycerol. Ketamine was purchased from Parke Davis (Ketalar, Hampshire, United Kingdom).
3.8.2. Induction of Sepsis and Drug Injection
Ketamine analogues were reconstituted in saline solution to a concentration of 1.25 mg per mL. Sepsis was induced in mice by intra-peritoneal (i.p.) inoculation of a lethal dose of E. coli (3.6 × 109 colony forming unit). Prior to bacteria inoculation mice were injected subcutaneously with 200 µL of ketamine (10 mg/kg), its analogues (10 mg/kg) or saline.
3.8.3. Sera and Peritoneal Lavage Fluids Collection and Cytokine Detection
At 16 hours after E. coli inoculation, animals were anesthetized with sodium pentobarbital (i.p., 50 mg/kg). A 1 mL syringe flushed with heparin was used to draw intra-cardial blood sample. The samples were stored on ice before centrifugation at 1,000 g at 40 °C for 10 minutes. The cell-free supernatants were collected and frozen at −200 °C for future analysis. Peritoneal lavage was performed with 5 mL phosphate buffer saline (PBS; Biological Industries, Beit Haemek, Israel) containing 2% bovine serum albumin (BSA; Sigma, Rehovot, Israel) and 5 mM ethylenediaminetetraacetic acid (EDTA; Sigma, Rehovot, Israel). After centrifugation at 400 g for 10 minutes, the cell-free supernatants were removed and frozen at −20 °C until analysis. TNFα and IL-6 levels were determined by commercial ELISA kits (Biolegend, San Diego, CA, USA and R&D Systems, Minneapolis, MN, USA, respectively).
3.9. Statistical Analysis
Results are expressed as mean ± S.E.M. To compare levels between treated groups and saline group, one-way analysis of variance was used. P values of <0.05 were considered significant.