Synthesis, Radiolabeling and Biological Evaluation of Propylene Amine Oxime Complexes Containing Nitrotriazoles as Hypoxia Markers
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis

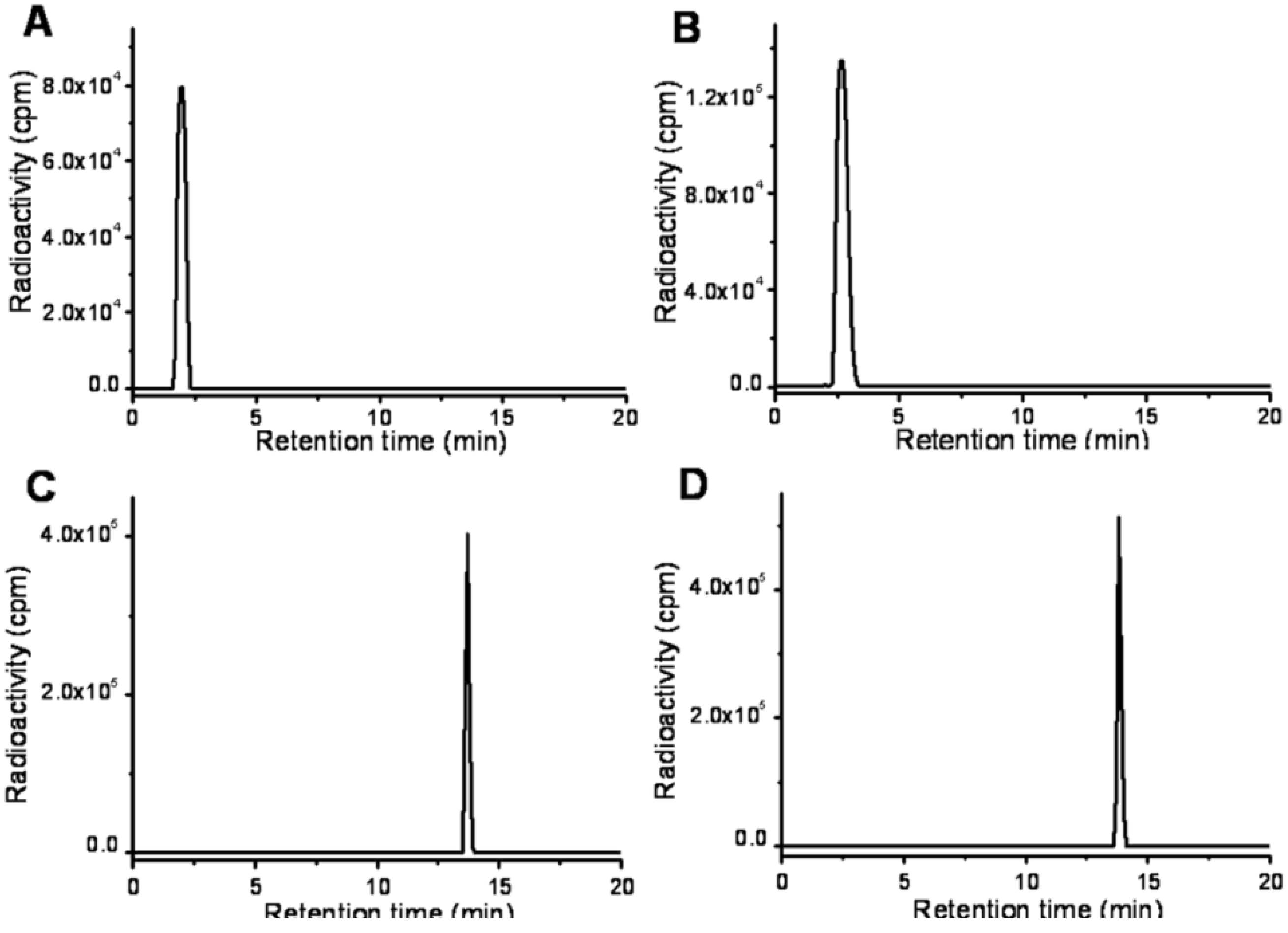
2.2. Radiolabeling
2.3. Octanol/Water Partition Coefficient
2.4. In Vitro Study


2.5. Biodistribution Study
| Tissue | 0.5 h | 1 h | 2 h | 4 h |
|---|---|---|---|---|
| Blood | 2.61 ± 0.65 | 1.97 ± 0.72 | 1.32 ± 0.40 | 0.91 ± 0.37 |
| Heart | 0.57 ± 0.13 | 0.43 ± 0.04 | 0.28 ± 0.02 | 0.24 ± 0.03 |
| Lung | 1.91 ± 0.74 | 1.30 ± 0.12 | 1.21 ± 0.23 | 0.83 ± 0.12 |
| Liver | 12.26 ± 5.14 | 8.15 ± 0.59 | 7.41 ± 2.03 | 4.91 ± 0.55 |
| Spleen | 0.52 ± 0.05 | 0.43 ± 0.05 | 0.37 ± 0.03 | 0.30 ± 0.05 |
| Stomach | 1.47 ± 0.36 | 1.42 ± 0.27 | 1.51 ± 0.19 | 1.16 ± 0.18 |
| Kidney | 1.73 ± 0.42 | 1.41 ± 0.42 | 1.17 ± 0.13 | 0.89 ± 0.16 |
| Muscle | 0.19 ± 0.03 | 0.16 ± 0.05 | 0.11 ± 0.02 | 0.09 ± 0.01 |
| Brain | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| Tumor | 0.47 ± 0.08 | 0.45 ± 0.01 | 0.42 ± 0.05 | 0.35 ± 0.03 |
| T/B | 0.16 ± 0.03 | 0.26 ± 0.10 | 0.34 ± 0.12 | 0.39 ± 0.19 |
| T/M | 2.46 ± 0.29 | 2.72 ± 0.55 | 3.88 ± 0.40 | 3.79 ± 0.59 |
| Tissue | 0.5 h | 1 h | 2 h | 4 h |
|---|---|---|---|---|
| Blood | 1.30 ± 0.14 | 1.17 ± 0.11 | 0.75 ± 0.38 | 0.84 ± 0.25 |
| Heart | 0.38 ± 0.13 | 0.29 ± 0.04 | 0.24 ± 0.08 | 0.18 ± 0.03 |
| Lung | 0.57 ± 0.06 | 0.49 ± 0.14 | 0.41 ± 0.09 | 0.31 ± 0.03 |
| Liver | 15.90 ± 2.06 | 18.77 ± 3.03 | 13.80 ± 3.58 | 9.09 ± 2.40 |
| Spleen | 0.31 ± 0.02 | 0.32 ± 0.09 | 0.29 ± 0.09 | 0.15 ± 0.02 |
| Stomach | 2.30 ± 1.42 | 1.54 ± 0.30 | 1.00 ± 0.11 | 0.75 ± 0.27 |
| Kidney | 1.85 ± 0.16 | 1.58 ± 0.28 | 1.42 ± 0.15 | 0.92 ± 0.15 |
| Muscle | 0.22 ± 0.04 | 0.12 ± 0.01 | 0.08 ± 0.02 | 0.05 ± 0.01 |
| Brain | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Tumor | 0.39 ± 0.06 | 0.34 ± 0.08 | 0.27 ± 0.06 | 0.25 ± 0.06 |
| T/B | 0.31 ± 0.03 | 0.30 ± 0.04 | 0.47 ± 0.29 | 0.31 ± 0.08 |
| T/M | 1.80 ± 0.39 | 2.87 ± 0.91 | 3.35 ± 0.60 | 4.58 ± 0.76 |
3. Experimental
3.1. General
3.2. Synthesis
3.3. Radiolabeling
3.4. Octanol/Water Partition Coefficient
3.5. In Vitro Study
3.6. Biodistribution Study
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds mentioned are available from the authors.
Reference and Notes
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C.A. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Nunn, A.; Linder, K.; Strauss, H.W. Nitroimidazoles and imaging hypoxia. Eur. J. Nucl. Med. 1995, 22, 265–280. [Google Scholar] [CrossRef]
- Rasey, J.S.; Koh, W.J.; Evans, M.L.; Peterson, L.M.; Lewellen, T.K.; Graham, M.M.; Krohn, K.A. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: A pretherapy study of 37 patients. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 417–428. [Google Scholar] [CrossRef]
- Saita, K.; Chen, M.; Spratt, N.J.; Porritt, M.J.; Liberatore, G.T.; Read, S.J.; Levi, C.R.; Donnan, G.A.; Ackermann, U.; Tochon-Danguy, H.J.; et al. Imaging the ischemic penumbra with 18F-fluoromisonidazole in a rat model of ischemic stroke. Stroke 2004, 35, 975–980. [Google Scholar]
- Martin, G.V.; Caldwell, J.H.; Graham, M.M.; Grierson, J.R.; Kroll, K.; Cowan, M.J.; Lewellen, T.K.; Rasey, J.S.; Casciari, J.J.; Krohn, K.A. Noninvasive detection of hypoxic myocardium using fluorine-18-fluoromisonidazole and positron emission tomography. J. Nucl. Med. 1992, 33, 2202–2208. [Google Scholar]
- Das, T.; Banerjee, S.; Samuel, G.; Sarma, H.D.; Korde, A.; Venkatesh, M.; Pillai, M.R.A. 99mTc-labeling studies of a modified metronidazole and its biodistribution in tumor bearing animal models. Nucl. Med. Biol. 2003, 30, 127–134. [Google Scholar] [CrossRef]
- Mallia, M.B.; Mathur, A.; Subramanian, S.; Banerjee, S.; Sarma, H.D.; Venkatesh, M. A novel [99mTc≡N]2+ complex of metronidazole xanthate as a potential agent for targeting hypoxia. Bioorg. Med. Chem. Lett. 2005, 15, 3398–3401. [Google Scholar] [CrossRef]
- Dobrowsky, W.; Huigol, N.G.; Jayatilake, R.S.; Kizilbash, N.I.A.; Okkan, S.; Kagiya, V.T.; Tatsuzaki, H. AK-2123 (Sanazol) as a radiation sensitizer in the treatment of stage III cervical cancer: Results of an IAEA multicentre randomised trial. Radiother. Oncol. 2007, 82, 24–29. [Google Scholar] [CrossRef]
- Huilgol, N.G.; Chatterjee, N.; Mehta, A.R. An overview of the initial experience with AK-2123 as a hypoxic cell sensitizer with radiation in the treatment of advanced head and neck cancers. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 1121–1124. [Google Scholar] [CrossRef]
- Sugie, C.; Shibamoto, Y.; Ito, M.; Ogino, H.; Suzuki, H.; Uto, Y.; Nagasawa, H.; Hori, H. Hori, H. Reevaluation of the radiosensitizing effects of sanazole and nimorazole in vitro and in vivo. J. Radiat. Res. 2005, 46, 453–459. [Google Scholar] [CrossRef]
- Murugesan, S.; Shetty, S.J.; Noronha, O.P.D.; Samuel, A.M.; Srivastava, T.S.; Nair, C.K.; Kothari, L. Technetium-99m-cyclam AK 2123: A novel marker for tumor hypoxia. Appl. Radiat. Isot. 2001, 54, 81–88. [Google Scholar] [CrossRef]
- Das, T.; Chakraborty, S.; Banerjee, S.; Mukherjee, A.; Samuel, G.; Sarma, H.D.; Nair, C.K.K.; Kagiya, V.T.; Venkatesh, M. Preparation and preliminary biological evaluation of a 177Lu labeled sanazole derivative for possible use in targeting tumor hypoxia. Bioorg. Med. Chem. 2004, 12, 6077–6084. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, T.; Gao, X.; Liu, X.; Yang, Z.; Guo, Z.; Wang, X. Synthesis and preliminary biological evaluation of the 99mTc labeled nitrobenzoimidazole and nitrotriazole as tumor hypoxia markers. Bioorg. Med. Chem. Lett. 2006, 16, 1831–1833. [Google Scholar] [CrossRef]
- Bejot, R.; Kersemans, V.; Kelly, C.; Carroll, L.; King, R.C.; Gouverneur, V. Pre-clinical evaluation of a 3-nitro-1,2,4-triazole analogue of [18F]FMISO as hypoxia-selective tracer for PET. Nucl. Med. Biol. 2010, 37, 565–575. [Google Scholar] [CrossRef]
- Jurisson, S.S.; Lydon, J.D. Potential technetium small molecule radiopharmaceuticals. Chem. Rev. 1999, 99, 2205–2218. [Google Scholar] [CrossRef]
- Linder, K.E.; Chan, Y.W.; Cyr, J.E.; Malley, M.F.; Nowotnik, D.P.; Nunn, A.D. TcO(PnAO-1-(2-nitroimidazole)) [BMS-181321], a new technetium-containing nitroimidazole complex for imaging hypoxia: synthesis, characterization, and xanthine oxidase-catalyzed reduction. J. Med. Chem. 1994, 37, 9–17. [Google Scholar] [CrossRef]
- Ballinger, J.R.; Kee, J.W.M.; Rauth, A.M. In vitro and in vivo evaluation of a technetium-99m-labeled 2-nitroimidazole (BMS181321) as a marker of tumor hypoxia. J. Nucl. Med. 1996, 37, 1023–1031. [Google Scholar]
- Melo, T.; Duncan, J.; Ballinger, J.R.; Rauth, A.M. BRU59–21, a second-generation 99mTc-labeled 2-nitroimidazole for imaging hypoxia in tumors. J. Nucl. Med. 2000, 41, 169–176. [Google Scholar]
- Huang, H.; Zhou, H.; Li, Z.; Wang, X.; Chu, T. Effect of a second nitroimidazole redox centre on the accumulation of a hypoxia marker: Synthesis and in vitro evaluation of 99mTc-labeled bisnitroimidazole propylene amine oxime complexes. Bioorg. Med. Chem. Lett. 2012, 22, 172–177. [Google Scholar] [CrossRef]
- Kung, H.F.; Yu, C.C.; Billings, J.; Molnar, M.; Blau, M. Synthesis of New bis(aminoethanethiol) (BAT) derivatives: Possible ligands for 99mTc brain imaging agents. J. Med. Chem. 1985, 28, 1280–1284. [Google Scholar] [CrossRef]
- Mastrostamatis, S.G.; Papadopoulos, M.S.; Pirmettis, I.C.; Paschali, E.; Varvarigou, A.D.; Stassinopoulou, C.I.; Raptopoulou, C.P.; Terzis, A.; Chiotellis, E. Tridentate ligands containing the SNS donor atom set as a novel backbone for the development of technetium brain-imaging agents. J. Med. Chem. 1994, 37, 3212–3218. [Google Scholar]
- Edwards, D.I. Nitroimidazole drugs—Action and resistance mechanisms. 1. Mechanisms of action. J. Antimicrob. Chemother. 1993, 31, 9–20. [Google Scholar] [CrossRef]
- Larina, L.; Lopyrev, V. Nitroazoles: Synthesis, Structure and Applications, 1st ed; Springer Press: New York, NY, USA, 2009; p. 284. [Google Scholar]
- Ramalingam, K.; Raju, N.; Nanjappan, P.; Nowotnik, D.P. Synthesis of nitroimidazole substituted 3,3,9,9-tetramethyl-4,8-diazaundecane-2,10-dione dioximes (propylene amine oximes, PnAOs): Ligands for technetium-99m complexes with potential for imaging hypoxic tissue. Tetrahedron 1995, 51, 2875–2894. [Google Scholar] [CrossRef]
- Dearling, J.L.J.; Lewis, J.S.; Mullen, G.E.D.; Rae, M.T.; Zweit, J.; Blower, P.J. Design of hypoxia-targeting radiopharmaceuticals: Selective uptake of copper-64 complexes in hypoxic cells in vitro. Eur. J. Nucl. Med. 1998, 25, 788–792. [Google Scholar] [CrossRef]
- Dearling, J.L.J.; Lewis, J.S.; Mullen, G.E.D.; Welch, M.J.; Blower, P.J. Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships. J. Biol. Inorg. Chem. 2002, 7, 249–259. [Google Scholar] [CrossRef]
- Zhang, X.G.; Melo, T.; Rauth, A.M.; Ballinger, J.R. Cellular accumulation and retention of the technetium-99m-labelled hypoxia markers BRU59–21 and butylene amine oxime. Nucl. Med. Biol. 2001, 28, 949–957. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, H.; Mei, L.; Chu, T. Synthesis, Radiolabeling and Biological Evaluation of Propylene Amine Oxime Complexes Containing Nitrotriazoles as Hypoxia Markers. Molecules 2012, 17, 6808-6820. https://doi.org/10.3390/molecules17066808
Huang H, Mei L, Chu T. Synthesis, Radiolabeling and Biological Evaluation of Propylene Amine Oxime Complexes Containing Nitrotriazoles as Hypoxia Markers. Molecules. 2012; 17(6):6808-6820. https://doi.org/10.3390/molecules17066808
Chicago/Turabian StyleHuang, Huafan, Lei Mei, and Taiwei Chu. 2012. "Synthesis, Radiolabeling and Biological Evaluation of Propylene Amine Oxime Complexes Containing Nitrotriazoles as Hypoxia Markers" Molecules 17, no. 6: 6808-6820. https://doi.org/10.3390/molecules17066808




