Effect of Frozen Storage on Molecular Weight, Size Distribution and Conformation of Gluten by SAXS and SEC-MALLS
Abstract
:1. Introduction
2. Results and Discussion
2.1. SAXS Analysis

| Sample | RgSAXS (nm) | I(0) (a.u) | Shape * | Chi2 |
|---|---|---|---|---|
| Control gluten | 12.84 ± 0.38 | (2.74 ± 0.14) × 105 | Globular | 2.180 |
| 30 days frozen | 9.71 ± 0.35 | (2.061 ± 0.082) × 105 | Globular | 0.7119 |
| 60 days frozen | 8.24 ± 0.25 | (1.705 ± 0.052) × 105 | Globular | 1.107 |
| 90 days frozen | 6.98 ± 0.19 | (1.494 ± 0.041) × 105 | Globular | 1.970 |
| 120 days frozen | 6.21 ± 0.19 | (1.225 ± 0.038) × 105 | Globular | 1.311 |

2.2. SEC-MALLS Analysis
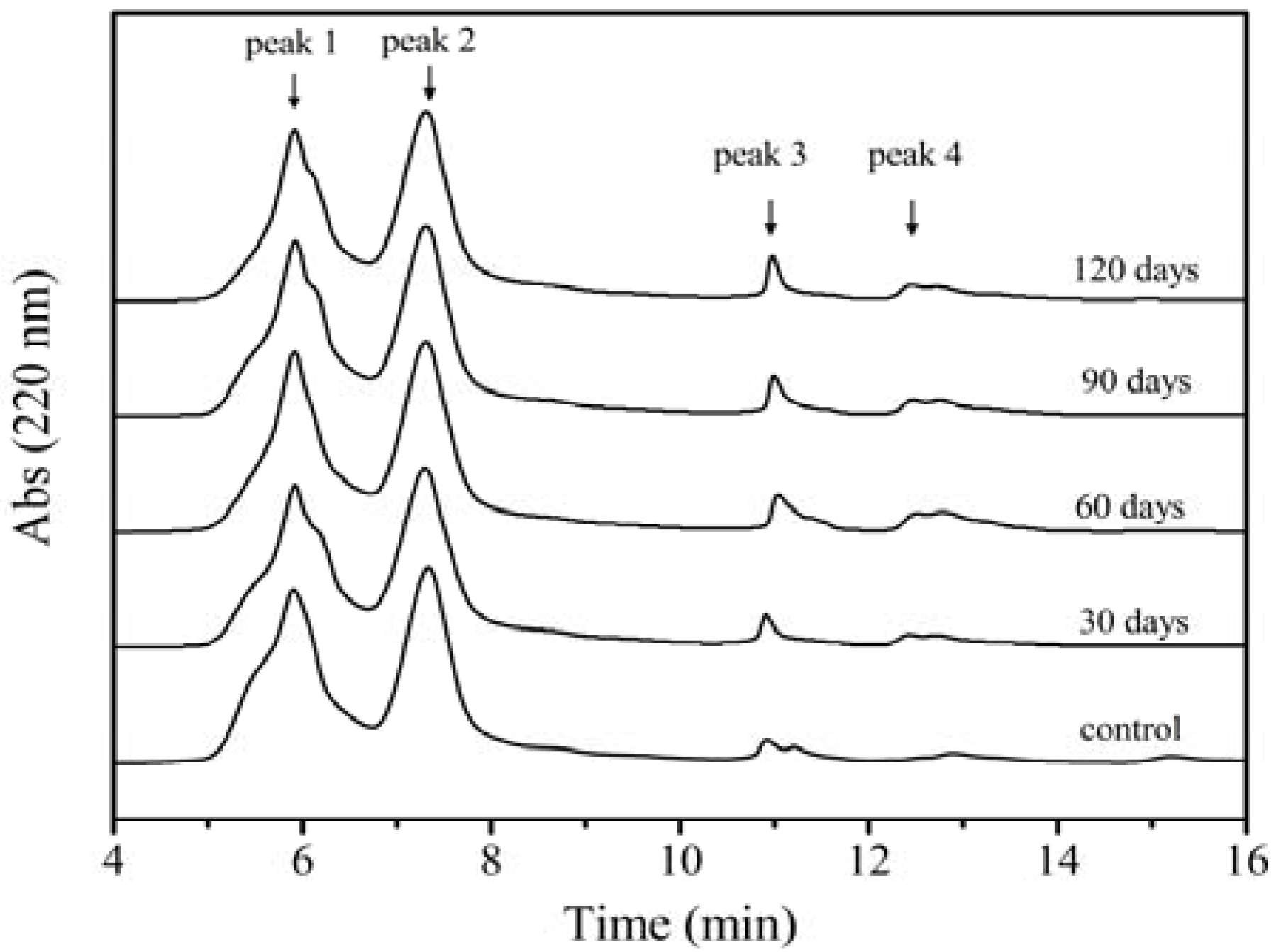

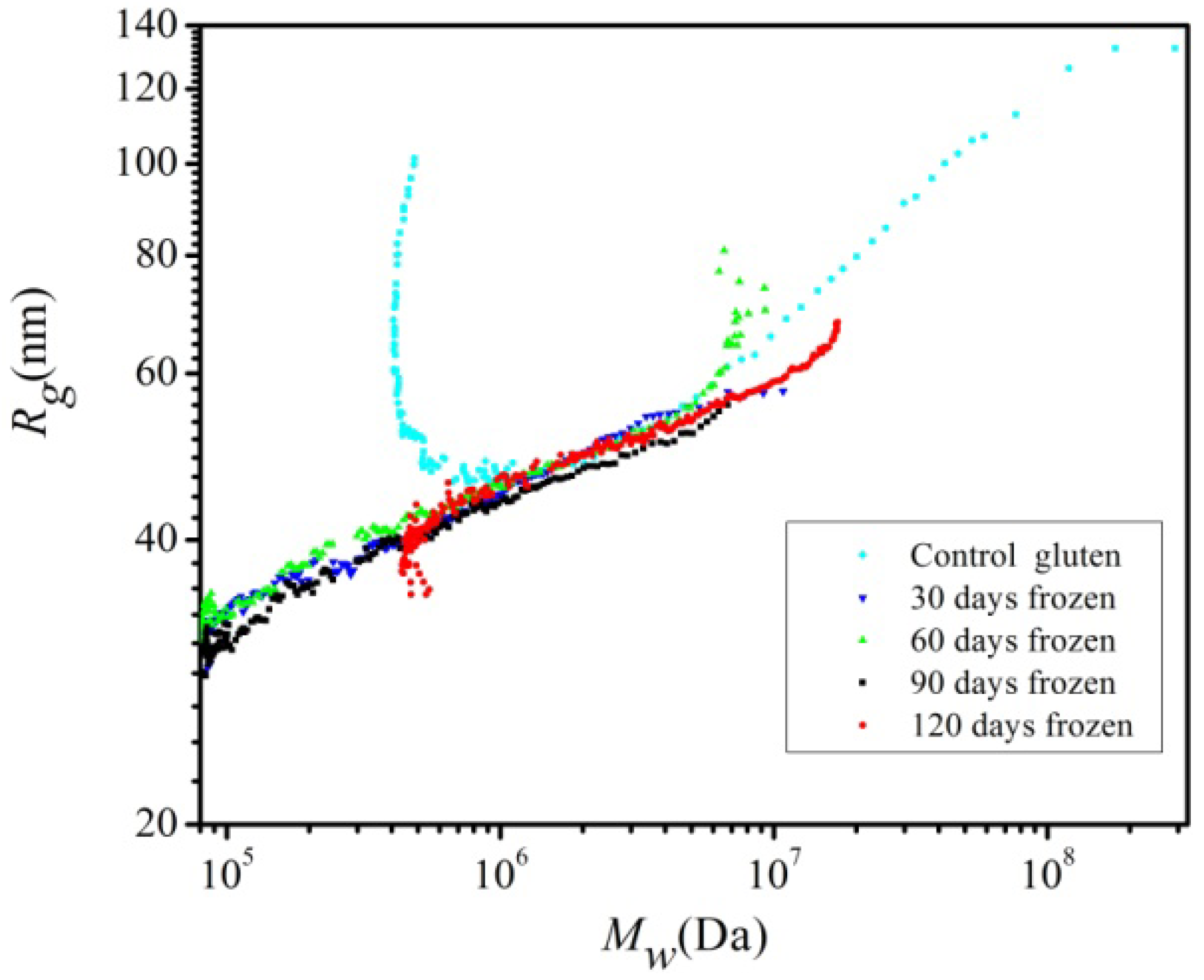

2.3. Effect of Storage Time on Free Amino Groups

2.4. Effect of Storage Time on the Free SH Groups Content of Wheat Gluten

3. Experimental
3.1. Materials
3.2. Wheat Gluten Extraction and Freezing
3.3. Gluten Solution Preparation
3.4. SAXS Measurement
3.5. SEC-MALLS Measurement
3.6. Quantification of Amino Groups
3.7. Free Sulfhydryl (SH) Determination
4. Conclusions
Acknowledgments
References and Notes
- Southan, M.; MacRitchie, F. Molecular weight distribution of wheat proteins. Cereal Chem. 1999, 76, 827–836. [Google Scholar] [CrossRef]
- Kennedy, C. Managing Frozen Foods; Woodhead Publishing Limited and CRC Press LLC: Cambridge, UK, 2000; pp. 143–149. [Google Scholar]
- Ribotta, P.; León, A.; Anon, M. Effect of freezing and frozen storage of doughs on bread quality. J. Agric. Food Chem. 2001, 49, 913–918. [Google Scholar] [CrossRef]
- Kuktaite, R.; Larsson, H.; Johansson, E. Variation in protein composition of wheat flour and its relationship to dough mixing behaviour. J. Cereal Sci. 2004, 40, 31–39. [Google Scholar] [CrossRef]
- Gupta, R.B.; Popineau, Y.; Lefebvre, J.; Cornec, M.; Lawrene, G.J.; Macritchie, F. Biochemical basis of flour properties in bread wheats. Ⅱ. changes in polymeric protein formation and dough/gluten properties associated with the loss of low Mr or high Mr glutenin subunits. J. Cereal Sci. 1995, 21, 103–116. [Google Scholar]
- Oliveira, C.L.P.; Behrens, M.A.; Pedersen, J.S.; Erlacher, K.; Otzen, D. A SAXS study of glucagon fibrillation. J. Mol. Biol. 2009, 387, 147–161. [Google Scholar] [CrossRef]
- Watanabe, Y.; Inoko, Y. Size-exclusion chromatography combined with small-angle X-ray scattering optics. J. Chromatogr. A 2009, 1216, 7461–7465. [Google Scholar]
- Egelhaaf, S.U.; Swieten, E.; Bosma, T.; Boef, E.; Dijk, A.A.; Robillard, G.T. Size and shape of the repetitive domain of high molecular weight wheat gluten proteins. I. Small-angle neutron scattering. Biopolymers 2003, 69, 311–324. [Google Scholar]
- Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chi. Acta 1993, 272, 1–40. [Google Scholar] [CrossRef]
- Bean, S.; Lookhart, G. Factors Influencing the Characterization of Gluten Proteins by Size-Exclusion Chromatography and Multiangle Laser Light Scattering (SEC-MALLS). Cereal Chem. 2001, 78, 608–618. [Google Scholar] [CrossRef]
- Mendichi, R.; Fisichella, S.; Savarino, A. Molecular weight, size distribution and conformation of Glutenin from different wheat cultivars by SEC-MALLS. J. Cereal Sci. 2008, 48, 486–493. [Google Scholar] [CrossRef]
- Kataoka, M.; Head, J.F.; Seaton, B.A.; Engelman, D.M. Melittin binding causes a large calcium-dependent conformational change in calmodulin. Proc. Natl. Acad. Sci. USA 1989, 86, 6944–6948. [Google Scholar]
- Weegels, P.; Van de Pijpekamp, A.; Graveland, A.; Hamer, R.; Schofield, J. Depolymerisation and Re-polymerisation of Wheat Glutenin During Dough Processing. I. Relationships between Glutenin Macropolymer Content and Quality Parameters. J. Cereal Sci. 1996, 23, 103–111. [Google Scholar] [CrossRef]
- Weegels, P.; Hamer, R.; Schofield, J. Depolymerisation and re-polymerisation of wheat glutenin during dough processing. II. Changes in composition. J. Cereal Sci. 1997, 25, 155–163. [Google Scholar] [CrossRef]
- Lindsay, M.P.; Skerritt, J.H. The glutenin macropolymer of wheat flour doughs: Structure-function perspectives. Trends Food Sci. Technol. 1999, 10, 247–253. [Google Scholar] [CrossRef]
- Kataoka, M.; Nishii, I.; Fujisawa, T.; Ueki, T.; Tokunaga, F.; Goto, Y. Structural characterization of the molten globule and native states of apomyoglobin by solution X-ray scattering. J. Mol. Biol. 1995, 249, 215–228. [Google Scholar] [CrossRef]
- Glatter, O.; Kratky, O. Small Angle X-ray Scattering; Academic Press: London, UK, 1982; pp. 150–180. [Google Scholar]
- Makowski, L.; Rodi, D.Y.; Mandava, S.; Devarapalli, S.; Fischetti, R.F. Characterization of protein fold by wide-angle X-ray solution scattering. J. Mol. Biol. 2008, 383, 731–744. [Google Scholar] [CrossRef]
- Blobel, J.; Bernadó, P.; Svergun, D.I.; Tauler, R.; Pons, M. Low-Resolution structure of transient protein-protein complexes using small-angle X-ray scattering. J. Am. Chem. Soc. 2009, 131, 4378–4386. [Google Scholar]
- Jin, K.S.; Kim, D.Y.; Rho, Y.; Le, V.B.; Kwon, E.; Kim, K.K.; Ree, M. Solution structures of RseA and its complex with RseB. J. Synchro. Radiat. 2008, 15, 219–222. [Google Scholar] [CrossRef]
- Selomulyo, V.O.; Zhou, W. Frozen bread dough: Effects of freezing storage and dough improvers. J. Cereal Sci. 2007, 45, 1–17. [Google Scholar] [CrossRef]
- Wolt, M.; D'Appolonia, B. Factors involved in the stability of frozen dough. II. The effects of yeast type, flour type, and dough additives on frozen-dough stability. Cereal Chem. 1984, 61, 213–221. [Google Scholar]
- Wyatt, P. Combined differential light scattering with various liquid chromatography separation techniques. Biochem. Soc. Trans. 1991, 19, 485. [Google Scholar]
- Carceller, J.; Aussenac, T. Size characterisation of glutenin polymers by HPSEC-MALLS. J. Cereal Sci. 2001, 33, 131–142. [Google Scholar] [CrossRef]
- Bleukx, W.; Roels, S.; Delcour, J. On the presence and activities of proteolytic enzymes in vital wheat gluten. J. Cereal Sci. 1997, 26, 183–193. [Google Scholar] [CrossRef]
- Wadhawan, C.K. Fundamental studies on vitality of gluten for breadmaking. PhD Thesis, University of Manitoba, Winnipeg, MB, Canada, 1988. [Google Scholar]
- Redman, D. Softening of gluten by wheat proteases. J. Sci. Food Agric. 1971, 22, 75–78. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yonezawa, D. Wheat flour proteases and their action on gluten proteins in dilute acetic acid. Agric. Biol. Chem. 1982, 46, 767–773. [Google Scholar] [CrossRef]
- Wrigley, C. Giant proteins with flour power. Nature 1996, 381, 738–739. [Google Scholar] [CrossRef]
- Jiang, B.; Kontogiorgos, V.; Kasapis, S.; Douglas Goff, H. Rheological investigation and molecular architecture of highly hydrated gluten networks at subzero temperatures. J. Food Eng. 2008, 89, 42–48. [Google Scholar] [CrossRef]
- Nielsen, P.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Gujral, H.S.; Rosell, C.M. Functionality of rice flour modified with a microbial transglutaminase. J. Cereal Sci. 2004, 39, 225–230. [Google Scholar] [CrossRef]
- Veraverbeke, W.; Larroque, O.; Békés, F.; Delcour, J. In vitro polymerization of wheat glutenin subunits with inorganic oxidizing agents. I. Comparison of single-step and stepwise oxidations of high molecular weight glutenin subunits. Cereal Chem. 2000, 77, 582–588. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophs. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds re available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, L.; Li, L.; Liu, G.-Q.; Liu, X.-X.; Li, B. Effect of Frozen Storage on Molecular Weight, Size Distribution and Conformation of Gluten by SAXS and SEC-MALLS. Molecules 2012, 17, 7169-7182. https://doi.org/10.3390/molecules17067169
Zhao L, Li L, Liu G-Q, Liu X-X, Li B. Effect of Frozen Storage on Molecular Weight, Size Distribution and Conformation of Gluten by SAXS and SEC-MALLS. Molecules. 2012; 17(6):7169-7182. https://doi.org/10.3390/molecules17067169
Chicago/Turabian StyleZhao, Lei, Lin Li, Guo-Qin Liu, Xing-Xun Liu, and Bing Li. 2012. "Effect of Frozen Storage on Molecular Weight, Size Distribution and Conformation of Gluten by SAXS and SEC-MALLS" Molecules 17, no. 6: 7169-7182. https://doi.org/10.3390/molecules17067169




