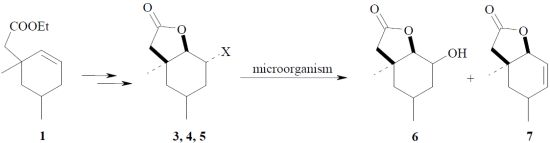
Fungal Strains as Catalysts for the Biotransformation of Halolactones by Hydrolytic Dehalogenation with the Dimethylcyclohexane System
Abstract
:1. Introduction
2. Results and Discussion

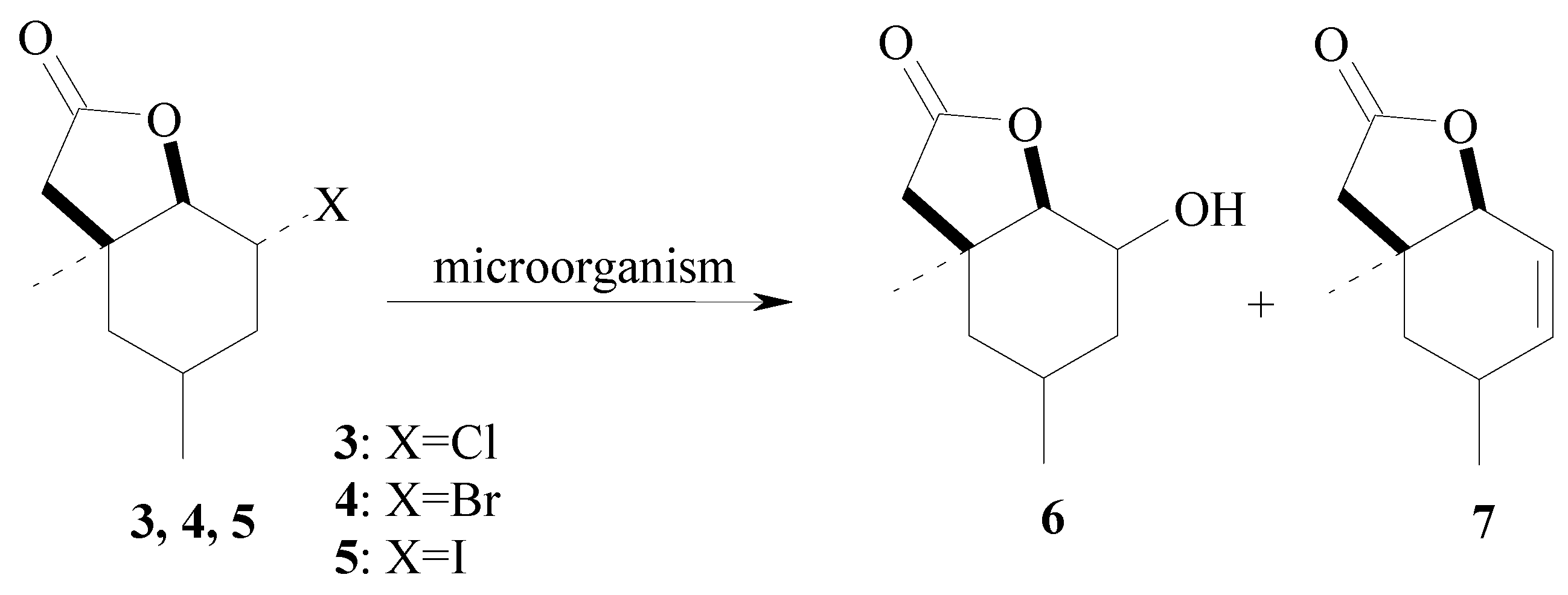
| Strain | Time of incubation (days) | Products of transformations (%) | |||||
|---|---|---|---|---|---|---|---|
| 6 (from 3) | 6 (from 4) | 6 (from 5) | 7 (from 5) | ||||
| Fusarium culmorum AM10 | 5 | - | 19.5 | 10.3 | 28.5 | ||
| 9 | 5.0 | 54.1 | 22.7 | 41.2 | |||
| 14 | 7.4 | 75.1 | 35.5 | 51.8 | |||
| Fusarium avenaceum AM11 | 5 | - | 7.8 | 4.8 | 1.4 | ||
| 9 | 27.4 | 48.2 | 10.4 | 2.9 | |||
| 14 | 57.7 | 56.3 | 15.9 | 6.3 | |||
| Fusarium oxysporum AM13 | 5 | 9.1 | 1.5 | 2.5 | - | ||
| 9 | 40.5 | 27.6 | 36.3 | - | |||
| 14 | 60.4 | 65.5 | 52.0 | - | |||
| Fusarium tricinctum AM16 | 5 | - | 10.8 | 3.1 | - | ||
| 9 | 20.9 | 24.7 | 16.4 | - | |||
| 14 | 53.3 | 52.4 | 24.8 | - | |||
| Fusarium semitectum AM20 | 5 | 5.7 | 6.6 | 10.5 | 11.0 | ||
| 9 | 16.7 | 30.3 | 22.7 | 20.0 | |||
| 14 | 51.4 | 55.9 | 42.0 | 9.3 | |||
| Fusarium solani AM203 | 5 | 1.8 | 27.7 | 2.7 | - | ||
| 9 | 33.0 | 48.7 | 35.8 | 6.5 | |||
| 14 | 76.6 | 61.1 | 54.7 | 7.7 | |||
| Botrytis cinerea AM235 | 5 | 3.3 | 3.6 | 7.3 | - | ||
| 9 | 84.1 | 56.6 | 24.3 | - | |||
| 14 | 85.3 | 95.0 | 27.7 | - | |||
| Beauveria bassiana AM278 | 5 | 6.1 | 3.3 | 3.5 | - | ||
| 9 | 22.3 | 18.7 | 10.2 | - | |||
| 14 | 29.7 | 26.5 | 19.7 | - | |||
| Strain | 3 (%) | 6 (%) | Isolated yield (%) | Isolated yield (g) | ee (%) | 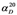 |
|---|---|---|---|---|---|---|
| F. avenaceum AM11 | 43.2 | 56.8 | 31.8 | 0.029 | 6.7 | +6.92 ( c = 1.06, CHCl3) |
| F. oxysporum AM13 | 39.0 | 61.0 | 26.3 | 0.024 | 15.8 | −7.45 ( c = 0.93, CHCl3) |
| F. tricinctum AM16 | 25.8 | 74.2 | 36.2 | 0.033 | 25.8 | −14.12 ( c = 0.60, CHCl3) |
| F. solani AM203 | 12.2 | 87.8 | 37.3 | 0.034 | 33.8 | −17.46 ( c = 0.59, CHCl3) |
| B. cinerea AM235 | 16.2 | 83.8 | 37.3 | 0.034 | 16.7 | −9.40 ( c = 0.32, CHCl3) |
| Strain | 4 (%) | 6 (%) | Isolated yield (%) | Isolated yield (g) | ee (%) |  |
|---|---|---|---|---|---|---|
| F. culmorum AM10 | 22.7 | 77.3 | 31.7 | 0.024 | 0 | - |
| F. avenaceum AM11 | 41.6 | 58.4 | 30.2 | 0.023 | 12.8 | −4.56 ( c = 0.59, CHCl3) |
| F. oxysporum AM13 | 33.0 | 67.0 | 28.6 | 0.021 | 19.0 | −9.44 ( c = 0.77, CHCl3) |
| F. semitectum AM20 | 37.3 | 62.7 | 43.0 | 0.032 | 16.2 | −7.11 ( c = 0.94, CHCl3) |
| F. solani AM203 | 33.7 | 66.3 | 38.0 | 0.028 | 32.0 | −33.42 ( c = 1.00, CHCl3) |
| B. cinerea AM235 | 7.3 | 92.7 | 59.0 | 0.044 | 8.9 | −2.20 ( c = 0.99, CHCl3) |
| Strain | 4 (%) | 6 (%) | 7 (%) | Isolated yield of 6 (%)/7 (%) | Isolated yield of 6 (g)/7 (g) | ee of 6 (%)/7 (%) | 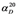 |
|---|---|---|---|---|---|---|---|
| F. culmorum AM10 | 15.2 | 39.9 | 44.9 | 5.8/10.9 | 0.004/0.006 | 15.3/0 | −15.83 ( c = 0.28, CHCl3)/- |
| F. oxysporum AM13 | 40.2 | 59.8 | - | 21.2/- | 0.016/- | 12.3/- | −11.22 ( c = 0.21, CHCl3) |
| F. solani AM203 | 37.4 | 55.5 | - | 11.1/- | 0.008/- | 25.1/- | −33.42 ( c = 0.38, CHCl3) |

3. Experimental
3.1. General
3.2. Microorganisms
3.3. Screening Biotransformation
3.4. Preparative Biotransformation
4. Conclusions
Acknowledgments
References
- Vairappan, C.S.; Suzuki, M.; Ishii, T.; Okino, T.; Abe, T.; Masuda, M. Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry 2008, 69, 2490–2494. [Google Scholar]
- Ji, N.Y.; Li, X.M.; Wang, B.G. Sesquiterpenes and Other Metabolites from the Marine Red Alga Laurencia composita (Rhodomelaceae). Helv. Chim. Acta 2010, 93, 2281–2286. [Google Scholar] [CrossRef]
- Kladi, M.; Vagias, C.; Papazafiri, P.; Furnari, G.; Serio, D.; Roussis, V. New sesquiterpenes from the red alga Laurencia microcladia. Tetrahedron 2007, 63, 7606–7611. [Google Scholar] [CrossRef]
- Su, H.; Shi, D.Y.; Li, J.; Guo, S.J.; Li, L.L.; Yuan, Z.H.; Zhu, X.B. Sesquiterpenes from Laurencia similes. Molecules 2009, 14, 1889–1897. [Google Scholar] [CrossRef]
- Rudi, A.; Benayahu, Y.; Kashman, Y. Negombins A-I, new chlorinated polyfunctional diterpenoids from the marine sponge Negombata species. Org. Lett. 2007, 9, 2337–2340. [Google Scholar] [CrossRef]
- Sung, P.J.; Chiang, M.Y.; Tsai, W.T.; Su, J.H.; Su, Y.M.; Wu, Y.C. Chlorinated briarane-type diterpenoids from the gorgonian coral Ellisella robusta (Ellisellidae). Tetrahedron 2007, 63, 12860–12865. [Google Scholar]
- Diaz-Marrero, A.R.; Brito, I.; de la Rosa, J.M.; D’Croz, L.; Fabelo, O.; Ruiz-Perez, C.; Darias, J.; Cueto, M. Novel lactone chamigrene-derived metabolites from Laurencia majuscule. Eur. J. Org. Chem. 2009, 1407–1411. [Google Scholar]
- Reed, K.A.; Manam, R.R.; Mitchell, S.S.; Xu, J.; Teisan, S.; Chao, T.H.; Deyanat-Yazdi, G.; Neuteboom, S.T.C.; Lam, K.S.; Potts, B.C.M. Salinosporamides D-J from the marine actinomycete Salinispora tropica, bromosalinosporamide, and thioester Derivatives are potent inhibitors of the 20S proteasome. J. Nat. Prod. 2007, 70, 269–276. [Google Scholar]
- Levenfors, J.J.; Hedman, R.; Thaning, C.; Gerhardson, B.; Welch, C.J. Broad-spectrum antifungal metabolites produced by the soil bacterium Serratia plymuthica A 153. Soil Biol. Biochem. 2004, 36, 677–685. [Google Scholar]
- Thaning, C.; Welch, C.J.; Borowicz, J.J.; Hedman, R.; Gerhardson, B. Suppression of Sclerotinia sclerotiorum apothecial formation by the soil bacterium Serratia plymuthica: Identification of a chlorinated macrolide as one of the causal agents. Soil Biol. Biochem. 2001, 33, 1817–1826. [Google Scholar] [CrossRef]
- Gutirrez-Cepeda, A.; Fernndez, J.J.; Gil, L.V.; Lopez-Rodriguez, M.; Norte, M.; Souto, M.L. Nonterpenoid C15 Acetogenins from Laurencia marilzae. J. Nat. Prod. 2011, 74, 441–448. [Google Scholar] [CrossRef]
- Nam, S.J.; Gaudncio, S.P.; Kauffman, C.A.; Jensen, P.R.; Kondratyuk, T.P.; Marler, L.E.; Pezzuto, J.M.; Fenical, W. Fijiolides A and B, inhibitors of TNF-α-induced NFκB activation, from a marine-derived sediment bacterium of the genus Nocardiopsis. J. Nat. Prod. 2010, 73, 1080–1086. [Google Scholar]
- Yin, S.; Boyle, G.M.; Carroll, A.R.; Kotiw, M.; Dearnaley, J.; Quinn, R.J.; Davis, R.A. Caelestines A−D, Brominated quinolinecarboxylic acids from the Australian ascidian Aplidium caelestis. J. Nat. Prod. 2010, 73, 1586–1589. [Google Scholar] [CrossRef]
- Lhullier, C.; Falkenberg, M.; Ioannou, E.; Quesada, A.; Papazafiri, P.; Horta, P.A.; Schenkel, E.P.; Vagias, C.; Roussis, V. Cytotoxic halogenated metabolites from the Brazilian red alga Laurencia catarinensis. J. Nat. Prod. 2010, 73, 27–32. [Google Scholar]
- Han, B.; McPhail, K.L.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Isolation and structure of five lyngbyabellin derivatives from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. Tetrahedron 2005, 61, 11723–11729. [Google Scholar]
- Zhang, L.; An, R.; Wang, J.; Sun, N.; Zhang, S.; Hu, J.; Kuai, J. Exploring novel bioactive compounds from marine microbes. Curr. Opin. Microbiol. 2005, 8, 276–281. [Google Scholar]
- Zidorn, C.; Ellmerer, E.P.; Konwalinka, G.; Schwaiger, N.; Stuppner, H. 13-Chloro-3-O-β-D-glucopyranosylsolstitialin from Leontodon palisae: The first genuine chlorinated sesquiterpene lactone glucoside. Tetrahedron Lett. 2004, 45, 3433–3436. [Google Scholar]
- Fehr, D.; Barlow, R.; McAtee, J.; Hemscheidt, T.K. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp. J. Nat. Prod. 2010, 73, 1963–1966. [Google Scholar] [CrossRef]
- Yang, X.; Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial bromotyrosine derivatives from the Australian marine sponge Hyattella sp. J. Nat. Prod. 2010, 73, 985–987. [Google Scholar] [CrossRef]
- Särkkä, J.; Paasivirta, J.; Häsänen, E.; Koistinen, J.; Manninen, P.; Mäntykoski, K.; Rantio, T.; Welling, L. Organic chlorine compounds in lake sediments. VI. Two bottom sites of Lake Ladoga near pulp mills. Chemosphere 1993, 26, 2147–2160. [Google Scholar] [CrossRef]
- Koistinen, J.; Herve, S.; Ruokojärvi, P.; Koponen, J.; Vartiainen, T. Persistent organic pollutants in two Finnish watercourses: Levels, congener profiles and source estimation by mussel incubation. Chemosphere 2010, 80, 625–633. [Google Scholar] [CrossRef]
- Papazi, A.; Kotzabasis, K. Bioenergetic strategy of microalgae for the biodegradation of phenolic compounds—Exogenously supplied energy and carbon sources adjust the level of biodegradation. J. Biotechnol. 2007, 129, 706–716. [Google Scholar]
- Papazi, A.; Kotzabasis, K. Inductive and resonance effects of substituents adjust the microalgal biodegradation of toxical phenolic compounds. J. Biotechnol. 2008, 135, 366–373. [Google Scholar] [CrossRef]
- Zanaroli, G.; Pérez-Jiménez, J.R.; Young, L.Y.; Marchetti, L.; Fava, F. Microbial Reductive Dechlorination of Weathered and Exogenous Co-planar Polychlorinated Biphenyls (PCBs) in an Anaerobic Sediment of Venice Lagoon. Biodegradation 2006, 17, 19–27. [Google Scholar]
- Hongsawat, P.; Vangnai, A.S. Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain. J. Hazard Mater. 2011, 186, 1300–1307. [Google Scholar] [CrossRef]
- Bao, H.; Gao, J.; Liu, Y.; Su, Y. A study of biodegradation/γ-irradiation on the degradation of p- chloronitrobenzene. Radiat. Phys. Chem. 2009, 78, 1137–1139. [Google Scholar]
- Katapodis, P.; Moukouli, M.; Christakopoulos, P. Biodegradation of indole at high concentration by persolvent fermentation with the thermophilic fungus Sporotrichum. Int. Biodeterior. Biodegradat. 2007, 60, 267–272. [Google Scholar] [CrossRef]
- Li, J.; Cai, W.; Zhu, L. The characteristics and enzyme activities of 4-chlorophenol biodegradation by Fusarium sp. Bioresour. Technol. 2011, 102, 2985–2989. [Google Scholar]
- Muzikar, M.; Kresinova, Z.; Svobodova, K.; Filipova, A.; Cvancarova, M.; Cajthamlova, K.; Cajthaml, T. Biodegradation of chlorobenzoic acids by ligninolytic fungi. J. Hazard. Mater. 2011, 196, 386–394. [Google Scholar] [CrossRef]
- Wang, C.; Xi, J.Y.; Hu, H.Y.; We, X.H. Biodegradation of Gaseous Chlorobenzene by White-rot Fungus Phanerochaete chrysosporium. Biomed. Enviro. Sci. 2008, 21, 474–478. [Google Scholar] [CrossRef]
- Hardman, D.J. Biotransformation of Halogenated Compounds. Crit. Rev. Biotechnol. 1991, 11, 1–40. [Google Scholar] [CrossRef]
- Janssen, D.B. Evolving haloalkane dehalogenases. Curr. Opin. Chem. Biol. 2004, 8, 150–159. [Google Scholar] [CrossRef]
- Janssen, D.B.; Oppentocht, J.E.; Poelarends, G.J. Microbial dehalogenation. Curr. Opin. Biotechnol. 2001, 12, 254–258. [Google Scholar] [CrossRef]
- Grotowska, A.K.; Wawrzeńczyk, C. Lactones 13. Biotransformation of iodolactones. J. Mol. Catal. B: Enzym. 2002, 19-20, 203–208. [Google Scholar]
- Grabarczyk, M.; Białońska, A. Biotransformations of chloro-, bromo- and iodolactone with trimethylcyclohexane system using fungal strains. Biocatal. Biotransform. 2010, 28, 408–414. [Google Scholar] [CrossRef]
- Gładkowski, W.; Mazur, M.; Białońska, A.; Wawrzeńczyk, C. Lactones 35. Metabolism of iodolactones with cyclohexane ring in Absidia cylindrospora culture. Enzyme Microb. Technol. 2011, 48, 326–333. [Google Scholar]
- Borges, K.B.; Borges, W.S.; Durán-Patrón, R.; Pupo, M.T.; Bonato, P.S.; Collado, I.C. Stereoselective biotransformations using fungi as biocatalysts. Tetrahedron: Asymmetry 2009, 20, 385–397. [Google Scholar] [CrossRef]
- Rodrigues-Filho, E.; Magnani, R.F.; Xie, W.; Mirocha, C.J.; Pathre, S.V. Hydroxylation of the Labdane Diterpene Cupressic Acid by Fusarium graminearum. J. Braz. Chem. Soc. 2002, 13, 266–269. [Google Scholar]
- Ghoumari, H.; Benajiba, M.H.; Garcia-Granados, A.; Fernandez, A.; Martinez, A.; Rivas, F.; Arias, J.M. Biotransformations of ent-18-acetoxy-6-ketomanoyl oxides epimers at C-13 with filamentous fungi. Phytochemistry 2006, 67, 2294–2302. [Google Scholar] [CrossRef]
- Garcia-Granados, A.; Fernandez, A.; Gutierrez, M.C.; Martinez, A.; Quiros, R.; Rivas, F.; Arias, J.M. Biotransformation of ent-13-epi-manoyl oxides difunctionalized at C-3 and C-12 by filamentous fungi. Phytochemistry 2004, 65, 107–115. [Google Scholar]
- Tapia, A.A.; Vallejo, M.D.; Gouiric, S.C.; Feresin, G.E.; Rossomando, P.C.; Bustos, D.A. Hydroxylation of dehydroabietic acid by Fusarium species. Phytochemistry 1997, 46, 131–133. [Google Scholar]
- wizdor, A.; Kołek, T. Transformations of 4- and 17α-substituted testosterone analogues by Fusarium culmorum. Steroids 2005, 70, 817–824. [Google Scholar] [CrossRef]
- Kumari, G.N.K.; Masilamani, S.; Ganesh, M.R.; Aravind, S. Microbial transformation of zaluzanin-D. Phytochemistry 2003, 62, 1101–1104. [Google Scholar]
- Nobilec, E.; Aniol, M.; Wawrzeńczyk, C. Lactones 1. Hydroxylation of dihydro-β-campholenolactone by Fusarium culmorum. Tetrahedron 1994, 50, 10339–10344. [Google Scholar]
- Gładkowski, W.; Grabarczyk, M.; Konopka, M.; Wawrzeńczyk, C. Lactones 20: Biohydroxylation of saturated bicyclic γ-lactones with the substituted cyclohexane system. J. Mol. Catal. B Enzym. 2004, 29, 13–17. [Google Scholar]
- Kaplan, O.; Nikolaou, K.; Pisvejcova, A.; Martinkova, L. Hydrolysis of nitriles and amides by filamentous fungi. Enzyme Microb. Technol. 2006, 38, 260–264. [Google Scholar]
- Bartmańska, A.; Huszcza, E.; Tronina, T. Transformation of isoxanthohumol by fungi. J. Mol. Catal. B: Enzym. 2009, 61, 221–224. [Google Scholar]
- Marostica, M.R., Jr.; Pastore, G.M. Production of R-(+)- α-terpineol by the biotransformation of limonene from orange essential oil, using cassava waste water as medium. Food Chem. 2007, 101, 345–350. [Google Scholar] [CrossRef]
- Bicas, J.L.; de Quadros, C.P.; Neri-Numa, I.A.; Pastore, G.M. Integrated process for co-production of alkaline lipase and R-(+)-α-terpineol by Fusarium oxysporum. Food Chem. 2010, 120, 452–456. [Google Scholar]
- Grogan, G.J.; Holland, H.L. The biocatalytic reactions of Beauveria spp. J. Mol. Catal. B: Enzym 2000, 9, 1–32. [Google Scholar] [CrossRef]
- Buchanan, G.O.; Williams, L.A.D.; Reese, P.B. Biotransformation of cadinane sesquiterpenes by Beauveria bassiana ATCC 7159. Phytochemistry 2000, 54, 39–45. [Google Scholar]
- Buchanan, G.O.; Reese, P.B. Biotransformation of diterpenes and diterpene derivatives by Beauveria bassiana ATCC 7159. Phytochemistry 2001, 56, 141–151. [Google Scholar]
- Qiao, L.; Xie, D.; Liu, Q.; Zou, J.; Shen, Z.; Dai, J. Microbial transformation of lovastatin by Beauveria bassiana. Acta Pharm. Sinica B 2012, 2, 300–305. [Google Scholar] [CrossRef]
- Aleu, J.; Collado, I.C. Biotransformations by Botrytis species. J. Mol. Catal. B: Enzym 2001, 13, 77–93. [Google Scholar] [CrossRef]
- Daoubi, M.; Duran-Patron, R.; Hernandez-Galan, R.; Benharref, A.; James, R.; Hanson, J.R.; Collado, I.C. The role of botrydienediol in the biodegradation of the sesquiterpenoid phytotoxin botrydial by Botrytis cinerea. Tetrahedron 2006, 62, 8256–8261. [Google Scholar]
- Bustillo, A.J.; Aleu, J.; Hernández-Galán, R.; Collado, I.C. Biotransformation of the fungistatic compound (R)-(+)-1-(4′-chlorophenyl)propan-1-ol by Botrytis cinerea. J. Mol. Catal. B: Enzym 2003, 21, 267–271. [Google Scholar] [CrossRef]
- Paruch, E.; Ciunik, Z.; Nawrot, J.; Wawrzeńczyk, C. Lactones 9. Synthesis of terpenoid lactones-active insect antifeedants. J. Agric. Food Chem 2000, 48, 4973–4977. [Google Scholar]
- Paruch, E.; Nawrot, J.; Wawrzeńczyk, C. Lactones 11. Feeding-deterrent activity of some bi- and tricyclic terpenoid lactones. Pest Manag. Sci. 2001, 57, 776–780. [Google Scholar] [CrossRef]
- Gabryś, B.; Szczepanik, M.; Dancewicz, K.; Szumny, A.; Wawrzeńczyk, C. Environmentally Safe Insect Control: Feeding Deterrent Activity of Alkyl-Substituted γ- and δ-Lactones to Peach Potato Aphid (Myzus persicae [Sulz.]) and Colorado Potato Beetle (Leptinotarsa decemlineata Say). Polish J. Environ. Stud 2006, 15, 549–556. [Google Scholar]
- Wawrzeńczyk, C.; Grabarczyk, M.; Szumny, A.; Gabryś, B.; Dancewicz, K.; Nawrot, J.; Prądzyńska, A.; Halarewicz-Pacan, A. Lactones 19. Synthesis and antifeedant activity of lactones with methyl-, dimethyl- and trimethylcyclohexane system. In Chemistry for Agriculture; Górecki, H., Dobrzański, Z., Kafarski, P., Eds.; Czech-Pol Trade: Wrocław, Poland, 2003; pp. 117–129. [Google Scholar]
- Szczepanik, M.; Grabarczyk, M.; Szumny, A.; Wawrzeńczyk, C. Feeding detergent activity of lactones with di- and trimethylcyclohexane system against lesser mealworm, Alphitobius diaperinus Panzer and Colorado potato beetle (Leptinotarsa decemlineata Say). J. Plant Prot. Res. 2003, 43, 87–96. [Google Scholar]
- Dancewicz, K.; Gabryś, B.; Halarewicz-Pacan, A.; Grabarczyk, M.; Wawrzeńczyk, C. Effect of terpenoid lactones with di- and trimethylcyclohexane systems on the behaviour of green peach aphid Myzus persicae. Pesticydy (Pesticides) 2005, 4, 17–23. [Google Scholar]
- Grabarczyk, M.; Szumny, A.; Gładkowski, W.; Białońska, A.; Ciunik, Z.; Wawrzeńczyk, C. Lactones 18. Synthesis of bicyclic lactones with methyl-, di- and trimethyl substituted cyclohexane system. Pol. J. Chem 2005, 79, 1763–1771. [Google Scholar]
- Sample Availability: Samples of the compounds 3–7 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grabarczyk, M. Fungal Strains as Catalysts for the Biotransformation of Halolactones by Hydrolytic Dehalogenation with the Dimethylcyclohexane System. Molecules 2012, 17, 9741-9753. https://doi.org/10.3390/molecules17089741
Grabarczyk M. Fungal Strains as Catalysts for the Biotransformation of Halolactones by Hydrolytic Dehalogenation with the Dimethylcyclohexane System. Molecules. 2012; 17(8):9741-9753. https://doi.org/10.3390/molecules17089741
Chicago/Turabian StyleGrabarczyk, Małgorzata. 2012. "Fungal Strains as Catalysts for the Biotransformation of Halolactones by Hydrolytic Dehalogenation with the Dimethylcyclohexane System" Molecules 17, no. 8: 9741-9753. https://doi.org/10.3390/molecules17089741