A Comparative Analysis of the Influence of Human Salivary Enzymes on Odorant Concentration in Three Palm Wines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Potent Wine Odorants
| Compound a | Odour-quality b | Retention FFAP | Index SE-54 | FD factor | RW | EW | BW | RWs | EWs | BWs | Previously identified in palm wine c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | Pungent fruity | nd | 500 | 4 | - | - | + | - | - | + | |
| Ethyl acetate | Fruity | 889 | 624 | 8 | - | - | + | - | - | - | |
| 2-Methyl butanal | Malty | 912 | 663 | 16 | + | + | - | - | - | - | |
| 3-Methyl butanal | Malty | 927 | 652 | 16 | + | + | + | - | - | - | |
| Methyl butanoate | Sweet fruity | 981 | 723 | 16 | - | + | - | - | + | - | |
| 2,3-Butandione | Buttery | 993 | 592 | 16 | + | + | + | + | + | + | [21,22] |
| Ethyl-2-methylbutanoate | Fruity | 1040 | 852 | 16 | + | + | + | + | + | + | |
| Ethyl pentanoate | Sweet fruity | 1067 | 900 | 16 | - | + | - | - | + | - | [22] |
| 2-Heptanone | Soapy fruity | 1181 | 891 | 8 | - | - | + | - | - | + | |
| 2/3-Methylbutanol | Malty | 1213 | 738 | 64 | + | + | + | + | + | + | |
| Ethyl hexanoate | Fruity | 1226 | 1001 | 64 | + | + | + | + | + | + | [21] |
| Acetoin | Buttery | 1275 | nd | 256 | + | + | + | + | + | + | |
| Ethyl lactate | Phenolic/smoky | 1321 | nd | 8 | + | + | - | - | - | - | |
| 2-Acetyl-1-pyrroline d | Popcorn | 1323 | 922 | 256 | + | + | + | + | + | + | |
| 3-Hydroxy-2-butanone | Buttery | nd | 718 | 8 | - | - | + | - | - | + | |
| 1-Hexanol | Rubbery | 1356 | 872 | 4 | + | + | + | + | + | + | |
| Acetic acid | Sweaty | 1428 | 600 | 16 | + | + | + | + | + | + | [21,22] |
| Ethyl octanoate | Fruity | 1429 | 1199 | 8 | + | - | - | + | - | - | |
| Hexyl-3-methyl butanoate | Fruity | 1430 | nd | 16 | + | + | - | - | - | - | |
| 2-Ethyl 3,5-dimethylpyrazine | Roasty | 1451 | 1083 | 64 | + | + | - | + | + | + | |
| Methional | Cooked potato | 1460 | nd | 8 | + | + | + | + | + | + | [22] |
| 3-Isobutyl-2-methoxypyrazine | Earthy | 1517 | 1175 | 256 | + | + | + | + | + | + | |
| 3-Methylbutyl acetate | Banana | 1527 | 878 | 128 | + | + | - | + | + | - | |
| 2-Acetylpyridine | popcorn | 1532 | nd | 64 | + | + | + | + | + | + | |
| Linalool | Fresh-blooming | 1540 | 1103 | 64 | + | + | + | + | + | + | |
| 2-Methylpropanoic acid | Sweaty | 1563 | nd | 128 | + | + | + | + | + | + | |
| Butanoic acid | Sweaty-buttery | 1619 | 821 | 8 | + | + | - | + | + | - | |
| 2/3-Methylbutanoic acid | Sweaty | 1661 | 875 | 16 | + | + | + | + | + | + | |
| (z)-1-5-Octadien-3-one | Geranium-like | 1676 | 984 | 4 | + | + | + | + | + | + | |
| ( E,E)-2,4-Nonadienal | Fatty | 1718 | 1215 | 8 | - | + | - | - | + | - | |
| Pentanoic acid | Sweaty | 1720 | 911 | 16 | + | + | + | + | + | + | |
| 3-Methylthiol-1,1-propanal | Broth-like | 1723 | 903 | 8 | + | + | - | - | - | - | |
| 3-Mercapto-2-methylpentanone d | Gravy-meaty | 1742 | 883 | 16 | - | + | - | - | - | - | |
| β-Damascenone | Flowery | 1801 | 1389 | 16 | + | + | + | + | + | + | |
| 2-Methoxyphenol | Smoky | 1842 | 1089 | 64 | + | + | + | + | + | + | |
| 2-Phenylethanol | Honey | 1911 | 1117 | 128 | + | + | + | + | + | + | |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | Caramel-like | 2038 | 1070 | 16 | + | + | + | - | - | - | |
| 3-Methylpentanoic acid | Sweaty | 2040 | nd | 8 | + | + | + | + | + | + | |
| Homofuraneol | Apple-like | 2095 | nd | 16 | + | + | - | + | + | - | |
| Ethyl cinnamate | Sweet | 2167 | 1469 | 8 | - | + | - | - | - | - | |
| Sotolone | Spicy | 2190 | 1110 | 8 | + | + | + | + | + | + | |
| Diethyl succinate | Sweet-pineapple | 2390 | nd | 4 | + | + | - | - | - | - | |
| y-Dodecalactone | Fruity | 2424 | 1497 | 16 | + | + | + | - | - | - | |
| Phenylacetic acid | Honey | 2577 | 1262 | 64 | + | + | + | + | + | + | |
| Vanillin | Vanilla | 2601 | 1404 | 16 | + | + | - | - | - | - | |
| 4-Methoxymethylphenol | Phenolic | 2639 | nd | 8 | - | + | - | - | + | - |
| No | Compounds | RW | EW | BW | RWS | EWS | BWS | OAVs (RW) b | OAVs (EW) b | OAVs (BW) b | OTW (µg/L) c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3-Methyl butanol | 19127 ± 25 a | 18300 ± 34 b | 18109 ± 15 c | 19315 ± 12 a | 18360 ± 19 b | 18315 ± 15 b | 19.1 a | 18.3 b | 19.3 a | 1000 |
| 2 | Ethyl hexanoate | 61.9 ± 3.2 a | 52.2 ± 7.1 b | 48.4 ± 5.2 c | 53.2 ± 6.3 a | 41.5 ± 3.8 b | 39.7 ± 4.6 c | 61.9 a | 52.2 b | 48.4 c | 1 |
| 3 | Acetoin | 712100 ± 21 a | 663500 ± 15 b | 452120 ± 10 c | 527100 ± 25 a | 410500 ± 14 b | 235600 ± 12 c | 890.13 a | 829.4 b | 565.15 c | 800 |
| 4 | 2-Acetylpyrroline | 9.8 ± 0.2 b | 11.4 ± 0.1 a | 5.3 ± 0.1 c | 9.6 ± 0.1 b | 11.3 ± 0.2 a | 5.0 ± 0.1 c | 98 b | 114 a | 53c | 0.1 |
| 5 | 2-Acetylpyridin | 0.32 ± 0.0 b | 0.32 ± 0.0 b | 0.45 ± 0.0 a | 0.30 ± 0.0 b | 0.30 ± 0.0 b | 0.41 ± 0.0 a | nd | nd | nd | nd |
| 6 | 2-Ethyl-3,5-dimethylpyrazine | 0.25 ± 0.0 b | 0.47 ± 0.0 a | nd | 0.23 ± 0.0 b | 0.46 ± 0.0 a | nd | 1.56 b | 2.9 a | nd | 0.16 |
| 7 | 3-Isobutyl-2-methoxypyrazine | 10.9 ± 0.0 b | 12.0 ± 0.0 a | 9.7 ± 0.0 c | 11.2 ± 0.0 b | 12.5 ± 0.1 a | 9.9 ± 0.0 c | 2180 b | 2400 a | 1940 c | 0.005 |
| 8 | 3-Methylbutyl acetate | 70.12 ± 5.2 a | 61.72 ± 3.1 b | nd | 68.50 ± 4.3 a | 59.73 ± 3.0 b | nd | 79.7 a | 70.0 b | nd | 0.88 |
| 9 | Linalool | 8.74 ± 0.1 c | 11.22 ± 0.1 b | 13.60 ± 0.1 a | 8.72 ± 0.1 c | 11.0 ± 0.1 b | 13.30 ± 1.2 a | 1.46c | 1.9 b | 2.3 a | 6 |
| 10 | 2-Methylpropanoic acid | 1650 ± 11 b | 1680 ± 9.0 b | 1735 ± 10 a | 1560 ± 8.5 b | 1580 ± 5.0 b | 1640 ± 9.0 a | <1 | <1 | <1 | 8100 |
| 11 | 2-Methoxy phenol | 0.34 ± 0.0 b | 0.28 ± 0.0 c | 0.40 ± 0.0 a | 0.34 ± 0.0 b | 0.28 ± 0.0 c | 0.40 ± 0.0 a | <1 | <1 | <1 | 3 |
| 12 | 2-Phenyl ethanol | 6570 ± 10 a | 5880 ± 8.0 b | 4870 ± 10 c | 6120 ± 15 a | 5470 ± 8.2 b | 4380 ± 10 c | 6.57 a | 5.88 b | 4.87 c | 1000 |
| 13 | Phenylacetic acid | 365 ± 4.5 c | 417 ± 7.0 b | 520 ± 5.0 a | 350 ± 6.0 c | 402 ± 4.1 b | 506 ± 6.0 a | <1 | <1 | <1 | 10000 |
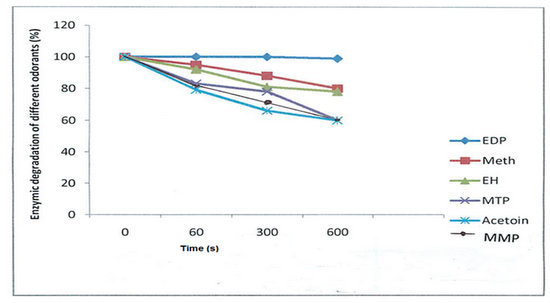
2.2. Influence of Saliva on the Palm Wine Odorants
2.2.1. Pyrazines and Alcohols
2.2.2. Aldehydes and Esters

2.2.3. Thiols

2.3. Odour Activity Values (OAVs)
3. Experimental
3.1. Materials
3.1.1. Raw Materials
3.2. Chemicals
3.3. Stable-Isotope-Labelled Standards
| No | Compounds a | Ions ( m/z) | Internal standard | Ion ( m/z) | Calibration factor b |
|---|---|---|---|---|---|
| 1 | 2-Methyl butanol | 71 | [2H3]-2-Methyl butanol | 74 | 0.88 |
| 2 | 3-Methyl butanol | 71 | [2H3]-2-Methyl butanol | 74 | 0.88 |
| 3 | Ethyl hexanoate | 145 | [2,2,2,-2H3]-Ethyl hexanoate | 148 | 1.0 |
| 4 | Acetoin | 91 | 13C2-Acetoin | 93 | 0.89 |
| 5 | 2-Acetylpyrroline | 60 | [2H3]-Acetylpyrroline | 61 | 1.0 |
| 6 | Acetic acid | 61 | [13C2]-Acetic acid | 63 | 1.0 |
| 7 | 2-Ethyl 3,5-dimethylpyrazine | 137 | 2-Ethyl [3,5-2H3] dimethylpyrazine | 140 | 1.0 |
| 8 | Methional | 105 | 3-([2H3]-Methylthiol)-1-propanal | 108 | 0.71 |
| 9 | 3-Isobutyl-2-methoxypyrazine | 167 | 3-Isobutyl-2-[2H3]-methoxypyrazine | 170 | 0.95 |
| 10 | 3-Methylbutyl acetate | 131 | 3-Methyl [3,4-2H3]-methoxypyrazine | 133 | 0.79 |
| 11 | 2-Acetylpyridine | 53 | 2-[2H3]-Acetylpyridine | 54 | 1.0 |
| 12 | Linalool | 137 | Tetrahydrolinalool | 141 | 1.61 |
| 13 | 2-Methoxyphenol | 125 | 2-[2H3]-Methoxyphenol | 128 | 1.0 |
| 14 | 2-Phenylethanol | 105 | 2-phenyl-[1,1,-2H2] ethanol | 107 | 1.0 |
| 15 | Phenylacetic acid | 137 | [13C2]-Phenylacetic acid | 139 | 1.0 |
| 16 | Vanillin | 151 | [2 H3]-Vanillin | 156 | 1.01 |
3.4. Enzymatic Analyses
3.4.1. Collection of Saliva and Enzyme Assay
3.4.2. Interaction of Wine with Saliva
3.4.3. Inhibition of Enzymatic Activity
3.5. Quantitation of the Odorants by Stable Isotope Dilution Assays
3.6. Chromatography-Mass Spectrometry
3.7. Aroma Extracts Dilution Analysis (AEDA)
3.8. Identification of Volatile Compounds
3.9. Calculation of Odour Activity Values (OAVs)
3.10. Statistical Treatment of Data
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Harrison, M. Effect of breathing and saliva flow on flavour release from liquid foods. J. Agric. Food Chem. 1998, 46, 2727–2735. [Google Scholar] [CrossRef]
- Mitropoulou, A.; Hatzidimitriou, E.; Paraskevopoulou, A. Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides and artificial saliva. Food Res. Int. 2011, 44, 1561–1570. [Google Scholar]
- Villamor, R.R.; Evans, M.A.; Mattinson, D.S.; Ross, C.F. Effects of ethanol, tannin and fructose on the headspace concentration and potential sensory significance of odorants in model wine. Food Res. Int. 2013, 50, 38–45. [Google Scholar]
- Camara, J.S.; Arminda, A.M.; Marques, J. Development of headspace solid-phase microextraction gas chromatography-mass spectrometry methodology for analysis of terpenoids in Madeira wines. Anal. Chim. Acta 2006, 555, 191–200. [Google Scholar]
- Lasekan, O.; Buettner, A.; Christlbauer, M. Investigation of the retro nasal perception of palm wine (Elaeis guineensis) aroma by application of sensory analysis and exhaled odorant measurement (EXOM). Afr. J. Food Agric. Nutr. Dev. 2009, 9, 793–813. [Google Scholar]
- Linforth, R.S.T.; Hodgson, M.; Taylor, A. Studies of Retronasal Flavour Delivery. Flavor Research at the Dawn of the Twenty-First Century. In Proceedings of the 10th Weurman Flavor Research Symposium, Beaune, France, 25–28 June 2003; Le Quere, J.L., Etievant, P.X., Eds.; pp. 143–147.
- Buettner, A. Investigation of Espresso ‘After Smell’ by the Use of the Buccal Odor Screening System (BOSS). In Proceedings of the 7th Wartburg Symposium on Flavor Chemistry and Biology, Eisenach Germany, 21–23 April 2004; Schieberle, P., Hofmann, T., Rothe, T., Eds.; pp. 167–178.
- Buettner, A.; Beer, A.; Hanning, C.; Settles, M.; Schieberle, P. Quantitation of the In-Mouth Release of Heteroatomic Odorants. Flavour Chemistry of Heteroatomic Compounds. In Proceedings of the ACS Symposium Series 826, Boston, USA, 22–23 July 2002; Reineccius, T., Reineccius, G., Eds.; America Chemical Society: Washington, DC, USA; pp. 296–311.
- Buettner, A.; Mestres, M. Investigation of the retronasal perception of strawberry aroma after smell depending on matrix composition. J. Agric. Food Chem. 2005, 54, 2716–2722. [Google Scholar]
- Buettner, A.; Griess, M. Retronasal Perception of Espresso Aroma. In Proceedings of the 7th Wartburg Symposium on Flavor Chemistry and Biology, Eisenach, Germany, 21–23 April 2004; Schieberle, P., Hofmann, T., Rothe, T., Eds.; pp. 210–218.
- Buettner, A. Investigation of potent odorants and afterodor development in two Chardonnay wines using the Buccal Odor Screening System (BOSS). J. Agric. Food Chem. 2004, 52, 2339–2346. [Google Scholar] [CrossRef]
- Buettner, A. Influence of human saliva on odorant concentrations: Part II: Aldehydes, alcohols, 3-alkyl-2-methoxypyrazine, methoxyphenols and hydroxyl-4,5-dimethyl-2 (5H)-furanone. J. Agric. Food Chem. 2002, 50, 7105–7110. [Google Scholar] [CrossRef]
- Taylor, A.J. Volatile flavour release from foods during eating. Crit. Rev. Food Sci. Nutr. 1996, 36, 765–784. [Google Scholar] [CrossRef]
- Buettner, A. The influence of human salivary enzymes on odorant Concentration changes occurringing in vivo; Part 1: Esters and thiols. J. Agric. Food Chem. 2002, 50, 3283–3289. [Google Scholar] [CrossRef]
- Buettner, A. Prolonged Retronasal Aroma Perception-A Phenomenon Influenced by Physiological Factors and Food Matrix Composition. Flavour Research at the Dawn of the Twenty-first Century. In Proceedings of the 10th Weurman Flavour Research Symposium, Beaune, France, 25–28 June 2003; Le Quere, J.L., Etievant, P.X., Eds.; pp. 188–193.
- Franzen, K.L.; Kinsella, J.E. Parameters affecting the binding of volatile flavour compounds in model food systems. 1. Proteins. J. Agric. Food Chem. 1974, 22, 675–678. [Google Scholar] [CrossRef]
- O’Neill, T.E. Flavour Binding by Food Proteins: An Overview. In Flavour-Food Interactions; McGorrin, R.J., Leleand, J.V., Eds.; American Chemical Society: Washington, DC, USA, 1996; pp. 59–74. [Google Scholar]
- Hussein, M.M.; Kachikian, R.; Pidel, A.R. Analysis for flavour residuals in the mouth by gas chromatography. J. Food Sci. 1983, 48, 1884–1885. [Google Scholar] [CrossRef]
- Friel, E.N.; Taylor, A.J. Effect of salivary components on volatile partitioning from solution. J. Agric. Food Chem. 2001, 49, 3898–3905. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Roozen, J.P. Influence of mastication and saliva on aroma release in a model mouth system. Food Chem. 2000a, 71, 339–345. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbaucer, G.; Fleischhacker, W.; Ngassoum, M.B. Analysis of the aroma compounds of two different palm wine species (‘matango’ and raffia) from Cameroon using SPME-GC-FID, SPME-GC-MS and olfactometry. Ernahrung Nutr. 2001, 25, 67–71. [Google Scholar]
- Uzochukwu, S.V.A.; Balogh, E.; Tucknott, O.G.; Lewis, M.J.; Ngoddy, P.O. Volatile constituents of palm wine and palm sap. J. Sci. Food Agric. 1994, 64, 405–411. [Google Scholar] [CrossRef]
- Sladek, N.E.; Manthey, C.L.; Maki, P.A.; Zhang, Z.; Landkamer, G.J. Xenobiotic oxidation catalysed by aldehyde dehydrogenases. Drug Metab. Rev. 1989, 20, 697–720. [Google Scholar] [CrossRef]
- Chauncey, H.H.; Lionetti, F.; Lisanti, V.F. Enzymes in human saliva. 1: The determination, distribution and origin of whole saliva enzymes. J. Dent. Res. 1954, 33, 321–334. [Google Scholar] [CrossRef]
- Genovese, A.; Piombino, P.; Gambuti, A.; Moio, L. Simulation of retro nasal aroma of white and red wine in a model mouth system. Investigating the influence of saliva on volatile compounds. Food Chem. 2009, 114, 100–107. [Google Scholar] [CrossRef]
- Svensson, B.E. Abilities of peroxidases to catalyse peroxidase-oxidase oxidation of thiols. Biochem. J. 1988, 256, 757–762. [Google Scholar]
- Svensson, B.E. Thiols as myeloperoxidase-oxidase substrates. Biochem. J. 1998, 253, 441–449. [Google Scholar]
- Semmelroch, P.; Laskawy, G.; Blank, I.; Grosch, W. Determination of potent odorants in roasted coffee by stable isotope dilution assays. Flav. Frag. J. 1995, 10, 1–7. [Google Scholar] [CrossRef]
- Schieberle, P. Primary odorants of pale lager beer. Differences to other beers and changes during storage. Zur Lebensm Undersuchungen Forsch 1991, 193, 558–565. [Google Scholar] [CrossRef]
- Guth, H.; Grosch, W. Quantitation of potent odorants of virgin olive oil by Stable isotope dilution assays. J. Am. Oil Chem. Soc. 1993, 70, 513–518. [Google Scholar] [CrossRef]
- Sen, A.; Laskawy, G.; Schieberle, P.; Grosch, W. Quantitative determination of beta-damascenone in foods using a stable isotope dilution assay. J. Agric. Food Chem. 1991, 39, 757–759. [Google Scholar] [CrossRef]
- Semmelroch, P.; Grosch, W. Studies on character impact odorants of coffee brews. J. Agric. Food Chem. 1996, 44, 537–543. [Google Scholar] [CrossRef]
- Buettner, A.; Schieberle, P. Evaluation of aroma difference between hand- squeezed juices from valencia late and Navel oranges by quantitation of key odorants and reconstitution experiments. J. Agric. Food Chem. 2001, 49, 2387–2394. [Google Scholar] [CrossRef]
- Lasekan, O.; Buettner, A.; Christlbauer, M. Investigation of important odorants of palm wine (Elaeis guineensis). Food Chem. 2007, 105, 15–23. [Google Scholar] [CrossRef]
- Schieberle, P. New Developments on Methods for Analysis of Volatile Flavour Compounds and Their Precursors. In Characterization of Food-Emerging Methods; Gaonkar, A.G., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1995; pp. 403–433. [Google Scholar]
- Sample Availability: Comercially available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lasekan, O. A Comparative Analysis of the Influence of Human Salivary Enzymes on Odorant Concentration in Three Palm Wines. Molecules 2013, 18, 11809-11823. https://doi.org/10.3390/molecules181011809
Lasekan O. A Comparative Analysis of the Influence of Human Salivary Enzymes on Odorant Concentration in Three Palm Wines. Molecules. 2013; 18(10):11809-11823. https://doi.org/10.3390/molecules181011809
Chicago/Turabian StyleLasekan, Ola. 2013. "A Comparative Analysis of the Influence of Human Salivary Enzymes on Odorant Concentration in Three Palm Wines" Molecules 18, no. 10: 11809-11823. https://doi.org/10.3390/molecules181011809