Synthesis and Antibacterial Evaluation of Some Novel Imidazole and Benzimidazole Sulfonamides
Abstract
:1. Introduction
2. Results and Discussion
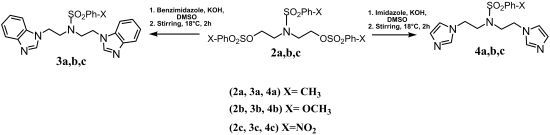
2.1. Synthesis





2.2. Antibacterial Activities
| No. | Structure of samples | Bacteria/MICs (mg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | ||||||||||
| Escherichia coli | Salmonella typhimurium | Pseudomonas aeruginosa | Acinetobacter calcoaceticus | Streptococcus pyogenes | Staphylococcus aureus | Bacillus subtilis | Rodococcus ruber | Enterococcus faecalis | Staphylococcus epidermidis | ||
| 3a | 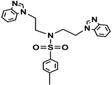 | >0.5 | 0.30 | >0.5 | >0.5 | 0.30 | 0.25 | 0.30 | 0.40 | 0.35 | 0.30 |
| 3b | 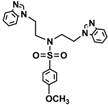 | 0.2 | >0.5 | >0.5 | 0.15 | 0.30 | 0.30 | 0.40 | 0.15 | 0.10 | >0.5 |
| 3c | 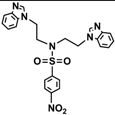 | 0.40 | 0.05 | 0.20 | 0.05 | 0.10 | 0.05 | 0.40 | 0.40 | >0.5 | 0.20 |
| 4a | 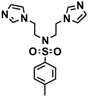 | >0.5 | 0.30 | >0.5 | >0.5 | 0.20 | 0.20 | 0.40 | 0.40 | 0.30 | 0.40 |
| 4b | 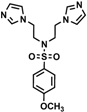 | 0.05 | 0.15 | 0.30 | >0.5 | 0.40 | 0.10 | 0.10 | 0.30 | 0.35 | >0.5 |
| 4c | 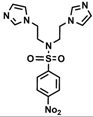 | >0.5 | 0.30 | >0.5 | 0.30 | 0.10 | 0.15 | 0.30 | 0.40 | >0.5 | 0.30 |
| 9 | 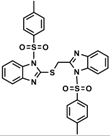 | >0.5 | >0.5 | 0.30 | 0.30 | 0.05 | 0.10 | 0.05 | 0.05 | >0.5 | 0.15 |
| 11 | 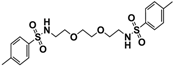 | 0.40 | 0.35 | >0.5 | >0.5 | 0.20 | 0.15 | 0.05 | 0.20 | 0.20 | 0.05 |
| AM |  | <0.05 | <0.05 | Nd | 0.15 | 0.05 | <0.05 | 0.25 | <0.05 | <0.05 | nd |
| KA |  | <0.05 | <0.05 | <0.05 | >0.5 | <0.05 | <0.05 | <0.05 | <0.05 | >0.5 | <0.05 |

3. Experimental
3.1. General
3.2. Synthesis N-(4-Methylbenzenesulfonyl)-bis((4-methylbenzenesulfonyl(oxy))ethyl)amine (2a) and N-(4-Methoxybenzenesulfonyl)-bis((4-methoxybenzenesulfonyl(oxy))-ethyl)amine (2b)
3.3. Synthesis N-(4-Nitrobenzenesulfonyl)-bis((4-nitrobenzenesulfonyl(oxy))ethyl)amine (2c)
3.4. General Procedure for Synthesis of 3a, 3b, 3c and 4a, 4b, 4c
3.5. Synthesis 2-Mercaptobenzimidazole (6)
3.6. Synthesis 2-((Benzimidazol-2-yl)methylthio)-benzimidazole (8)
3.7. Synthesis N-4-Methylbenzenesulfonyl ((N-(4-methylbenzenesulfonyl)benzimidazol-2-yl)methylthio)-benzimidazole (9)
3.8. Synthesis 4-Methyl-N-(2-{2-[2-(4-ethylbenzenesulfonamido)ethoxy]ethoxy}ethyl)benzenesulfon-amide (11)
3.9. Antibacterial Evaluation
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Zhang, H.; Li, X.; Chen, G.; Li, Q.S.; Luo, Y.; Ruan, B.F.; Chen, X.W.; Zhu, H.L. Design, synthesis and biological evaluation of pyridine acyl sulfonamide derivatives as novel COX-2 inhibitors. Bioorg. Med. Chem. 2011, 19, 6827–6832. [Google Scholar] [CrossRef]
- Luo, Y.; Qiu, K.M.; Lu, X.; Liu, K.; Fu, J.; Zhu, H.L. Synthesis, biological evaluation, and molecular modeling of cinnamic acyl sulfonamide derivatives as novel antitubulin agents. Bioorg. Med. Chem. 2011, 19, 4730–4738. [Google Scholar] [CrossRef]
- Chandak, N.; Bhardwaj, J.K.; Sharma, R.K.; Sharma, P.K. Inhibitors of apoptosis in testicular germ cells: Synthesis and biological evaluation of some novel IBTs bearing sulfonamide moiety. Eur. J. Med. Chem. 2013, 59, 203–208. [Google Scholar] [CrossRef]
- Kamal, A.; Swapna, P.; Shetti, R.V.; Shaik, A.B.; Narasimha Rao, M.P.; Gupta, S. Synthesis, biological evaluation of new oxazolidino-sulfonamides as potential antimicrobial agents. Eur. J. Med. Chem. 2013, 62, 661–669. [Google Scholar] [CrossRef]
- Akurathi, V.; Dubois, L.; Lieuwes, N.G.; Chitneni, S.K.; Cleynhens, B.J.; Vullo, D.; Supuran, C.T.; Verbruggen, A.M.; Lambin, P.; Bormans, G.M. Synthesis and biological evaluation of a 99mTc-labelled sulfonamide conjugate for in vivo visualization of carbonic anhydrase IX expression in tumor hypoxia. Nucl. Med. Biol. 2010, 37, 557–564. [Google Scholar] [CrossRef]
- Andrighetti-Fröhner, C.R.; de Oliveira, K.N.; Gaspar-Silva, D.; Pacheco, L.K.; Joussef, A.C.; Steindel, M.; Simões, C.M.O.; de Souza, A.M.T.; Magalhaes, U.O.; Afonso, I.F.; Rodrigues, C.R.; Nunes, R.J.; Castro, H.C. Synthesis, biological evaluation and SAR of sulfonamide 4-methoxychalcone derivatives with potential antileishmanial activity. Eur. J. Med. Chem. 2009, 44, 755–763. [Google Scholar] [CrossRef]
- Gadad, A.K.; Mahajanshetti, C.S.; Nimbalkar, S.; Raichurkar, A. Synthesis and antibacterial activity of some 5-guanylhydrazone/thiocyanato-6-arylimidazo[2,1-b]-1,3,4-thiadiazole-2-sulfonamide derivatives. Eur. J. Med. Chem. 2000, 35, 853–857. [Google Scholar] [CrossRef]
- Azab, M.; Youssef, M.; El-Bordany, E. Synthesis and antibacterial evaluation of novel heterocyclic compounds containing a sulfonamido moiety. Molecules 2013, 18, 832–844. [Google Scholar] [CrossRef]
- Ezabadi, I.R.; Camoutsis, C.; Zoumpoulakis, P.; Geronikaki, A.; Soković, M.; Glamočilija, J.; Ćirić, A. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg. Med. Chem. 2008, 16, 1150–1161. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; Arafa, R.K.; El-Hossary, E.M. In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido[4,5-b]quinolines bearing a sulfonamide moiety. Eur. J. Med. Chem. 2010, 45, 3677–3684. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Ragab, F.A.; Hamed, M.M. Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur. J. Med. Chem. 2009, 44, 4211–4217. [Google Scholar] [CrossRef]
- Bano, S.; Javed, K.; Ahmad, S.; Rathish, I.G.; Singh, S.; Alam, M.S. Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur. J. Med. Chem. 2011, 46, 5763–5768. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Johar, M.; Singhal, N.; Dastidar, S.G.; Shukla, R.; Raghubir, R. Synthesis and anticancer, antiinflammatory and analgesic activity evaluation of some sulfa drug and acridine derivatives. Monatsh. Chem. 2000, 131, 511–520. [Google Scholar] [CrossRef]
- El-Araby, M.; Omar, A.; Hassanein, H.H.; El-Helby, A.G.H.; Abdel-Rahman, A.A. Design, synthesis and in vivo anti-inflammatory activities of 2,4-diaryl-5-4H-imidazolone derivatives. Molecules 2012, 17, 12262–12275. [Google Scholar] [CrossRef]
- Nanthakumar, R.; Muthumani, P.; Girija, K. Anti-inflammatory and antibacterial activity study of some novel quinazolinones. Arab. J. Chem. 2011. [Google Scholar] [CrossRef]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, W.; Liu, K.; Yang, S.; Fan, H.; Bhadury, P.S.; Huang, D.Y.; Zhang, Y. Synthesis and antiviral activity of 5-(4-chlorophenyl)-1,3,4-thiadiazole sulfonamides. Molecules 2010, 15, 9046–9056. [Google Scholar] [CrossRef]
- Atia, A.J.K. Synthesis and antibacterial activities of new metronidazole and imidazole derivatives. Molecules 2009, 14, 2431–2446. [Google Scholar] [CrossRef]
- Kipp, B.H.; Faraj, C.; Li, G.; Njus, D. Imidazole facilitates electron transfer from organic reductants. Bioelectrochemistry 2004, 64, 7–13. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Soltani, R.M.N.; Mohabatkar, H.; Asrari, Z.; Hemmateenejad, B. Design, synthesis, antibacterial and QSAR studies of benzimidazole and imidazole chloroaryloxyalkyl derivatives. Bioorg. Med. Chem. 2005, 13, 1931–1938. [Google Scholar] [CrossRef]
- Jain, A.K.; Ravichandran, V.; Sisodiya, M.; Agrawal, R.K. Synthesis and antibacterial evaluation of 2-substituted-4,5-diphenyl-N-alkyl imidazole derivatives. Asian Pac. J. Trop. Med. 2010, 3, 471–474. [Google Scholar] [CrossRef]
- González-Chávez, M.M.; Méndez, F.; Martínez, R.; Pérez-González, C.; Martínez-Gutiérrez, F. Design and synthesis of anti-MRSA benzimidazolylbenzene-sulfonamides. QSAR studies for prediction of antibacterial activity. Molecules 2010, 16, 175–189. [Google Scholar] [CrossRef]
- Hernández-Núñez, E.; Tlahuext, H.; Moo-Puc, R.; Torres-Gómez, H.; Reyes-Martínez, R.; Cedillo-Rivera, R.; Nava-Zuazo, C.; Navarrete-Vazquez, G. Synthesis and in vitro trichomonicidal, giardicidal and amebicidal activity of N-acetamide(sulfonamide)-2-methyl-4-nitro-1H-imidazoles. Eur. J. Med. Chem. 2009, 44, 2975–2984. [Google Scholar] [CrossRef]
- Karakurt, A.; Dalkara, S.; Özalp, M.; Özbey, S.; Kendi, E.; Stables, J.P. Synthesis of some 1-(2-naphthyl)-2-(imidazole-1-yl)ethanone oxime and oxime ether derivatives and their anticonvulsant and antimicrobial activities. Eur. J. Med. Chem. 2001, 36, 421–433. [Google Scholar] [CrossRef]
- Suzuki, F.; Kuroda, T.; Tamura, T.; Soichiro, S.; Ohmori, K.; Ichikawa, S. New antiinflammatory agents. 2, 5-Phenyl-3H-imidazo[4,5-c][1,8]naphthyridin-4(5H)-ones: A new class of nonsteroidal antiinflammatory agents with potent activity like glucocorticoids. J. Med. Chem. 1992, 35, 2863–2870. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Isloor, A.M.; Telkar, S.; Arulmoli, T.; Fun, H.K. Molecular docking studies of some new imidazole derivatives for antimicrobial properties. Arab. J. Chem. 2013, 6, 197–204. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sharma, P.K.; Kumar, N. A review on “Imidazoles”: Their chemistry and pharmacological potentials. Int. J. PharmTech Res. 2011, 3, 268–282. [Google Scholar]
- Chak, B.C.; McAuley, A. The synthesis and characterization of the pendant-armed ligand N,N′-bis(2′-pyridylmethyl)-1,7-dithia-4,11-diazacyclotetradecane (L4) and crystal structures of L4 and the copper(II) complex [Cu(L4)](ClO4)2 Crystal structure of the nickel(II) complex of N-(2′-pyridylmethyl)-1,4,7-trithia-11-azacyclotetradecane (L2), [Ni(L2)(CH3CN)](ClO4)2·CH3CN. Can. J. Chem. 2006, 84, 187–195. [Google Scholar] [CrossRef]
- Starikova, O.V.; Dolgushin, G.V.; Larina, L.I.; Ushakov, P.E.; Komarova, T.N.; Lopyrev, V.A. Synthesis of 1,3-dialkylimidazolium and 1,3-dialkylbenzimidazolium salts. Russ. J. Org. Chem. 2003, 39, 1467–1470. [Google Scholar] [CrossRef]
- Al-Mohammed, N.N.; Alias, Y.; Abdullah, Z.; Khaledi, H. 1-Tosyl-2-[(1-tosyl-1H-benzimidazol-2-yl)methylsulfanyl]-1H-benzimidazole. Acta Crystallogr. Sect. E 2011, 67, o1043. [Google Scholar]
- Al-Mohammed, N.N.; Alias, Y.; Abdullah, Z.; Khaledi, H. N,N′-{[Ethane-1,2-diylbis(oxy)]bis- (ethane-2,1-diyl)}bis(4-methylbenzenesulfonamide). Acta Crystallogr. Sect. E 2012, 68, o1983. [Google Scholar] [CrossRef]
- Plech, T.; Wujec, M.; Siwek, A.; Kosikowska, U.; Malm, A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem. 2011, 46, 241–248. [Google Scholar] [CrossRef]
- Kossakowski, J.; Krawiecka, M.; Kuran, B.; Stefańska, J.; Wolska, I. Synthesis and preliminary evaluation of the antimicrobial activity of selected 3-benzofurancarboxylic acid derivatives. Molecules 2010, 15, 4737–4749. [Google Scholar] [CrossRef]
- Gu, W.; Wu, R.; Qi, S.; Gu, C.; Si, F.; Chen, Z. Synthesis and antibacterial evaluation of new N-acylhydrazone derivatives from dehydroabietic acid. Molecules 2012, 17, 4634–4650. [Google Scholar] [CrossRef]
- Cecil, R. The Role of Sulfur in Proteins. In The Proteins, 2nd ed.; Neurath, H., Ed.; Academic Press: New York, NY, USA, 1963; Volume 1, p. 379. [Google Scholar]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazol-2-ylthio]acetic acid arylidene-hydrazide derivatives. Farmaco 2001, 56, 587–592. [Google Scholar] [CrossRef]
- Mazzone, G.; Bonina, F.; Puglisi, G.; Reina, R.R.; Arrigo, C.; Cosentino, C.; Blandino, G. Synthesis and biological evaluation of some 5-aryl-2-amino-1,3,4-oxa(thia)diazoles. Farmaco 1982, 37, 685–700. [Google Scholar]
- Alwan, S.M. Synthesis and preliminary antimicrobial activities of new arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido cephalosporanic acids. Molecules 2012, 17, 1025–1038. [Google Scholar] [CrossRef]
- Greim, H.; Bury, D.; Klimisch, H.J.; Oeben-Negele, M.; Ziegler-Skylakakis, K. Toxicity of aliphatic amines: Structure-activity relationship. Chemosphere 1998, 36, 271–295. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, Y.; Zhao, Y.; He, G.; Xie, Y.; Liu, J.; He, J.; Liu, B.; Wei, Y. Preparation, antibacterial evaluation and preliminary structure–activity relationship (SAR) study of benzothiazol- and benzoxazol-2-amine derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3044–3049. [Google Scholar] [CrossRef]
- Matos, M.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Muñoz-Crego, A. Synthesis and structure-activity relationships of novel amino/nitro substituted 3-arylcoumarins as antibacterial agents. Molecules 2013, 18, 1394–1404. [Google Scholar] [CrossRef]
- Daniel, M.; Harry, L.; Mai, V. Guide to Antimicrobials; San Francisco VA Medical Center Infectious Diseases Section: San Francisco, CA, USA, 2012. [Google Scholar]
- Wang, M.L.; Liu, B.L. Synthesis of 2-mercaptobenzimidazole from the reaction of o-phenylene diamine and carbon disulfide in the presence of potassium hydroxide. J. Chinese Inst. Chem. Engineers 2007, 38, 161–167. [Google Scholar] [CrossRef]
- Satyanarayana, S.; Nagasundara, K.R. Synthesis and spectral properties of the complexes of cobalt(II), nickel(II), copper(II), zinc(II), and cadmium(II) with 2-(Thiomethyl-2′-benzimidazolyl)-benzimidazol. Synth. React. Inorg. Met. Org. Chem. 2004, 34, 883–895. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard M7-A7; Wayne, PA, USA, 2006; Volume 26.
- Sample Availability: Samples of the final compounds 3a–c, 4a–c, 9 and 11 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Mohammed, N.N.; Alias, Y.; Abdullah, Z.; Shakir, R.M.; Taha, E.M.; Hamid, A.A. Synthesis and Antibacterial Evaluation of Some Novel Imidazole and Benzimidazole Sulfonamides. Molecules 2013, 18, 11978-11995. https://doi.org/10.3390/molecules181011978
Al-Mohammed NN, Alias Y, Abdullah Z, Shakir RM, Taha EM, Hamid AA. Synthesis and Antibacterial Evaluation of Some Novel Imidazole and Benzimidazole Sulfonamides. Molecules. 2013; 18(10):11978-11995. https://doi.org/10.3390/molecules181011978
Chicago/Turabian StyleAl-Mohammed, Nassir N., Yatimah Alias, Zanariah Abdullah, Raied M. Shakir, Ekhlass M. Taha, and Aidil Abdul Hamid. 2013. "Synthesis and Antibacterial Evaluation of Some Novel Imidazole and Benzimidazole Sulfonamides" Molecules 18, no. 10: 11978-11995. https://doi.org/10.3390/molecules181011978
APA StyleAl-Mohammed, N. N., Alias, Y., Abdullah, Z., Shakir, R. M., Taha, E. M., & Hamid, A. A. (2013). Synthesis and Antibacterial Evaluation of Some Novel Imidazole and Benzimidazole Sulfonamides. Molecules, 18(10), 11978-11995. https://doi.org/10.3390/molecules181011978