Vascular Aldosterone Production at the Pre-Diabetic Stage of Young Otsuka Long-Evans Tokushima Fatty (OLETF) Rats, Compared with Long-Evans Tokushima Otsuka (LETO) Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. CYP11B2 mRNA Expression in Aortic SMC

2.2. Pregnenolone Production

2.3. Aldosterone Production
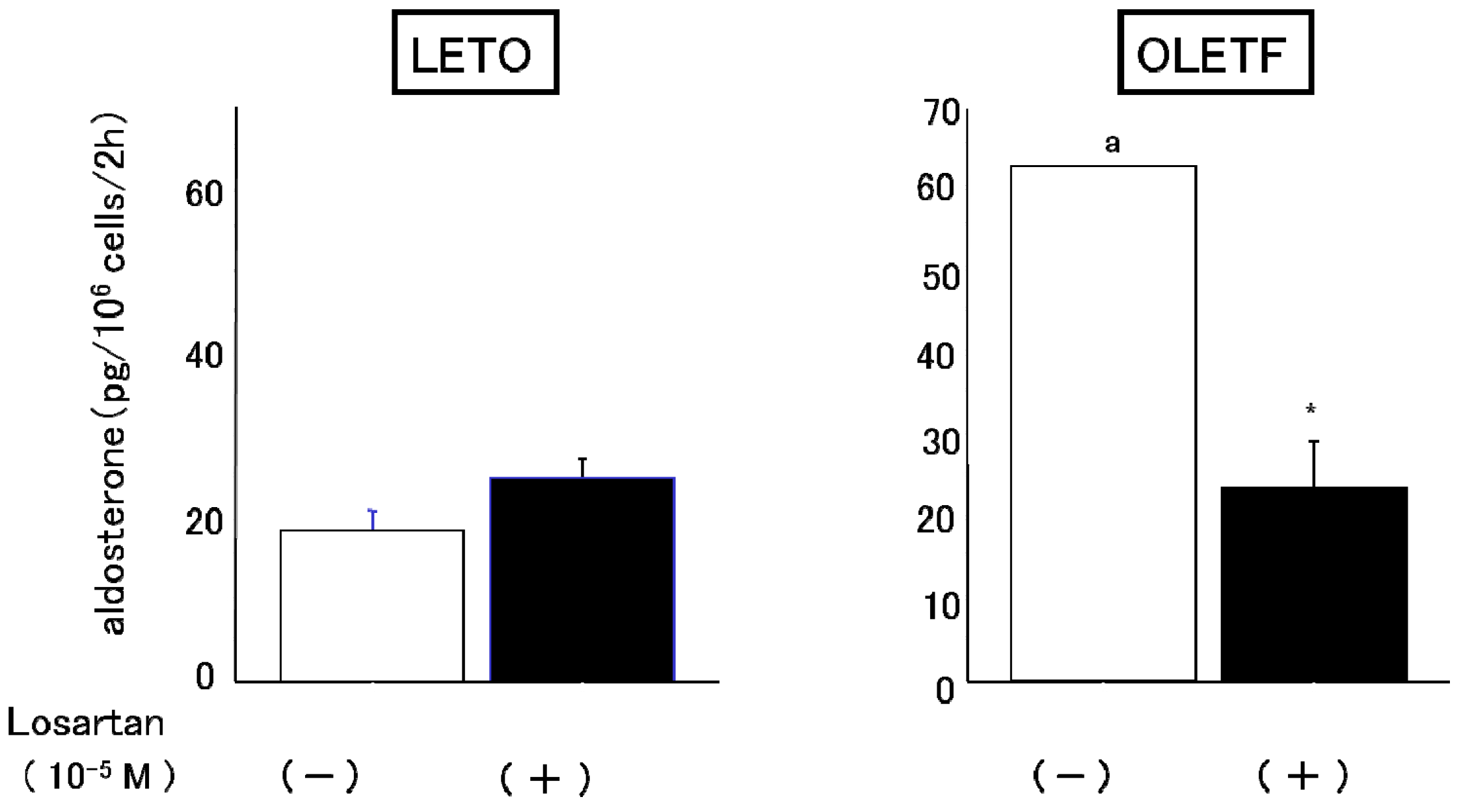
2.3.1. Effects of Angiotensin II and Losartan

2.3.2. Effects of HDL

2.4. AT1 Receptor Protein Expression
2.4.1. Effect of Angiotensin II
2.4.2. Effect of Angiotensin I


2.5. Discussion
3. Experimental
3.1. Materials
3.2. Animals
3.3. Preparation of Rat Aortic Smooth Muscle Cells
3.4. Cell Number Determination
3.5. Reverse Transcription and the Polymerase Chain Reaction
3.6. Pregnenolone Formation
3.7. Aldosterone Production
3.8. Immunoblotting of the Angiotensin II Receptor
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Takeda, Y. Vascular synthesis of aldosterone: Role in hypertension. Mol. Cell. Endocrinol. 2004, 31, 75–79. [Google Scholar] [CrossRef]
- Takeda, Y.; Miyamori, I.; Inaba, S.; Furukawa, K.; Hatakeyama, H.; Yoneda, T.; Mabuchi, H.; Takeda, R. Vascular aldosterone in genetically hypertensive rats. Hypertension 1997, 29, 45–48. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Miyamori, I.; Takeda, Y.; Yamamoto, H.; Mabuchi, H. The expression of steroidogenic enzyme genes in human vascular cells. Biochem. Mol. Biol. Int. 1996, 40, 639–645. [Google Scholar]
- Silvestre, J.S.; Robert, V.; Heymes, C.; Aupetit-Faisant, B.; Mouas, C.; Moalic, J.M.; Swynghedauw, B.; Delcayre, C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J. Biol. Chem. 1998, 273, 4883–4891. [Google Scholar] [CrossRef]
- Nishikawa, T.; Suematsu, S.; Saito, J.; Soyama, A.; Ito, H.; Kono, T.; Chrousos, G. Human renal mesangial cells produce aldosterone in response to low-density lipoprotein (LDL). J. Steroid Biochem. Mol. Biol. 2005, 96, 309–316. [Google Scholar] [CrossRef]
- Siragy, H.M.; Xue, C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp. Physiol. 2008, 93, 817–824. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.E.; Zhou, M.Y.; Cozza, E.N.; Morita, H.; Foecking, M.F.; Gomez-Sanchez, E.P. Aldosterone biosynthesis in the rat brain. Endocrinology 1997, 138, 3369–3373. [Google Scholar] [CrossRef]
- Bender, S.B.; McGraw, A.P.; Jaffe, I.Z.; Sowers, J.R. Mineralocorticoid receptor-mediated vascular insulin resistance: An early contributor to diabetes-related vascular disease? Diabetes 2013, 62, 313–319. [Google Scholar] [CrossRef]
- Briet, M.; Schiffrin, E.L. Vascular actions of aldosterone. J. Vasc. Res. 2013, 50, 89–99. [Google Scholar] [CrossRef]
- Nishikawa, T.; Matsuzawa, Y.; Suematsu, S.; Saito, J.; Omura, M.; Kino, T. Effect of atorvastatin on aldsterone production induced by glucose, LDL or angiotensin IIin human renal mesangial cells. Arzneim. Forsch. 2010, 60, 445–451. [Google Scholar]
- Hostetter, T.H.; Rosenberg, M.E.; Ibrahim, H.N.; Juknevicius, I. Aldosterone in renal disease. Curr. Opin. Nephrol. Hypertens. 2001, 10, 105–110. [Google Scholar] [CrossRef]
- Kawano, K.; Hirashima, T.; Mori, S.; Natori, T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: A new NIDDM rat strain. Diabetes Res. Clin. Pract. 1994, 24, 317–320. [Google Scholar] [CrossRef]
- Nemoto, S.; Taguchi, K.; Matsumoto, T.; Kamata, K.; Kobayashi, T. Pravastatin normalizes ET-1-induced contraction in the aorta of type 2 diabetic OLETF rats by suppressing the KSR1/ERK complex. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H893–H902. [Google Scholar] [CrossRef]
- Hosomi., H.; Noma, T.; Ohyama, H.; Takahashi, T.; Kohno, M. Vascular proliferation and transforming growth factor-β expression in pre-and early stage of diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis 2002, 162, 69–76. [Google Scholar] [CrossRef]
- Iizuka, K.; Machida, T.; Kawaguchi, H.; Hirafuji, M. Pulsatile mechanical pressure promotes angiotensin-converting enzyme expression in aortic smooth muscle cells. Cardiovasc. Drugs Ther. 2008, 22, 383–390. [Google Scholar] [CrossRef]
- Lavrentyev, E.N.; Estes, A.M.; Malik, K.U. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ. Res. 2007, 101, 455–464. [Google Scholar] [CrossRef]
- Saha, S.; Willenberg, H.S.; Bornstein, S.R.; Graessler, J.; Kopprasch, S. Diabetic lipoproteins and adrenal aldosterone synthesis—A possible pathophysiological link? Horm. Metab. Res. 2012, 44, 239–244. [Google Scholar] [CrossRef]
- Gwynne, J.T.; Mahaffee, D.D. Rat adrenal uptake and metabolism of high density lipoprotein cholesteryl ester. J. Biol. Chem. 1989, 264, 8141–8150. [Google Scholar]
- Nishikawa, T.; Mikami, K.; Yoshida, A.; Omura, M.; Tamura, Y.; Saito, Y. Regulation of cholesterol metabolism in adrenal cortex: Effects of apoproteins on cholesterol esterase in rat adrenal glands. Endocr. J. 1993, 40, 221–225. [Google Scholar] [CrossRef]
- Simpson, H.D.; Shepherd, R.; Shepherd, J.; Fraser, R.; Lever, A.F.; Kenyon, C.J. Effects of cholesterol and lipoproteins on aldosterone secretion by bovine zona glomerulosa cells. J. Endocrinol. 1989, 121, 125–131. [Google Scholar] [CrossRef]
- Xing, Y.; Cohen, A.; Rothblat, G.; Sankaranarayanan, S.; Weibel, G.; Royer, L.; Francone, O.L.; Rainey, W.E. Aldosterone production in human adrenocortical cells is stimulated by high-density lipoprotein 2 (HDL2) through increased expression of aldosterone synthase (CYP11B2). Endocrinology 2011, 152, 751–763. [Google Scholar] [CrossRef]
- Yaguchi, H.; Tsutsumi, K.; Shimono, K.; Omura, M.; Sasano, H.; Nishikawa, T. Involvement of high density lipoprotein as substrate cholestero1 for steroidogenesis by bovine adrenal fasciculo-reticu1aris cel1s. Life Sci. 1998, 62, 1387–1395. [Google Scholar] [CrossRef]
- Ross, R. Growth of smooth muscle in culture and formation of elastic fibers. J. Cell Biol. 1971, 50, 172–186. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matsuzawa, Y.; Suematsu, S.; Saito, J.; Omura, M.; Nishikawa, T. Vascular Aldosterone Production at the Pre-Diabetic Stage of Young Otsuka Long-Evans Tokushima Fatty (OLETF) Rats, Compared with Long-Evans Tokushima Otsuka (LETO) Rats. Molecules 2013, 18, 15636-15647. https://doi.org/10.3390/molecules181215636
Matsuzawa Y, Suematsu S, Saito J, Omura M, Nishikawa T. Vascular Aldosterone Production at the Pre-Diabetic Stage of Young Otsuka Long-Evans Tokushima Fatty (OLETF) Rats, Compared with Long-Evans Tokushima Otsuka (LETO) Rats. Molecules. 2013; 18(12):15636-15647. https://doi.org/10.3390/molecules181215636
Chicago/Turabian StyleMatsuzawa, Yoko, Sachiko Suematsu, Jun Saito, Masao Omura, and Tetsuo Nishikawa. 2013. "Vascular Aldosterone Production at the Pre-Diabetic Stage of Young Otsuka Long-Evans Tokushima Fatty (OLETF) Rats, Compared with Long-Evans Tokushima Otsuka (LETO) Rats" Molecules 18, no. 12: 15636-15647. https://doi.org/10.3390/molecules181215636
APA StyleMatsuzawa, Y., Suematsu, S., Saito, J., Omura, M., & Nishikawa, T. (2013). Vascular Aldosterone Production at the Pre-Diabetic Stage of Young Otsuka Long-Evans Tokushima Fatty (OLETF) Rats, Compared with Long-Evans Tokushima Otsuka (LETO) Rats. Molecules, 18(12), 15636-15647. https://doi.org/10.3390/molecules181215636



