Degradation of Methyl Blue Using Fe-Tourmaline as a Novel Photocatalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Degradation of MB by Fe-Tourmaline Powder
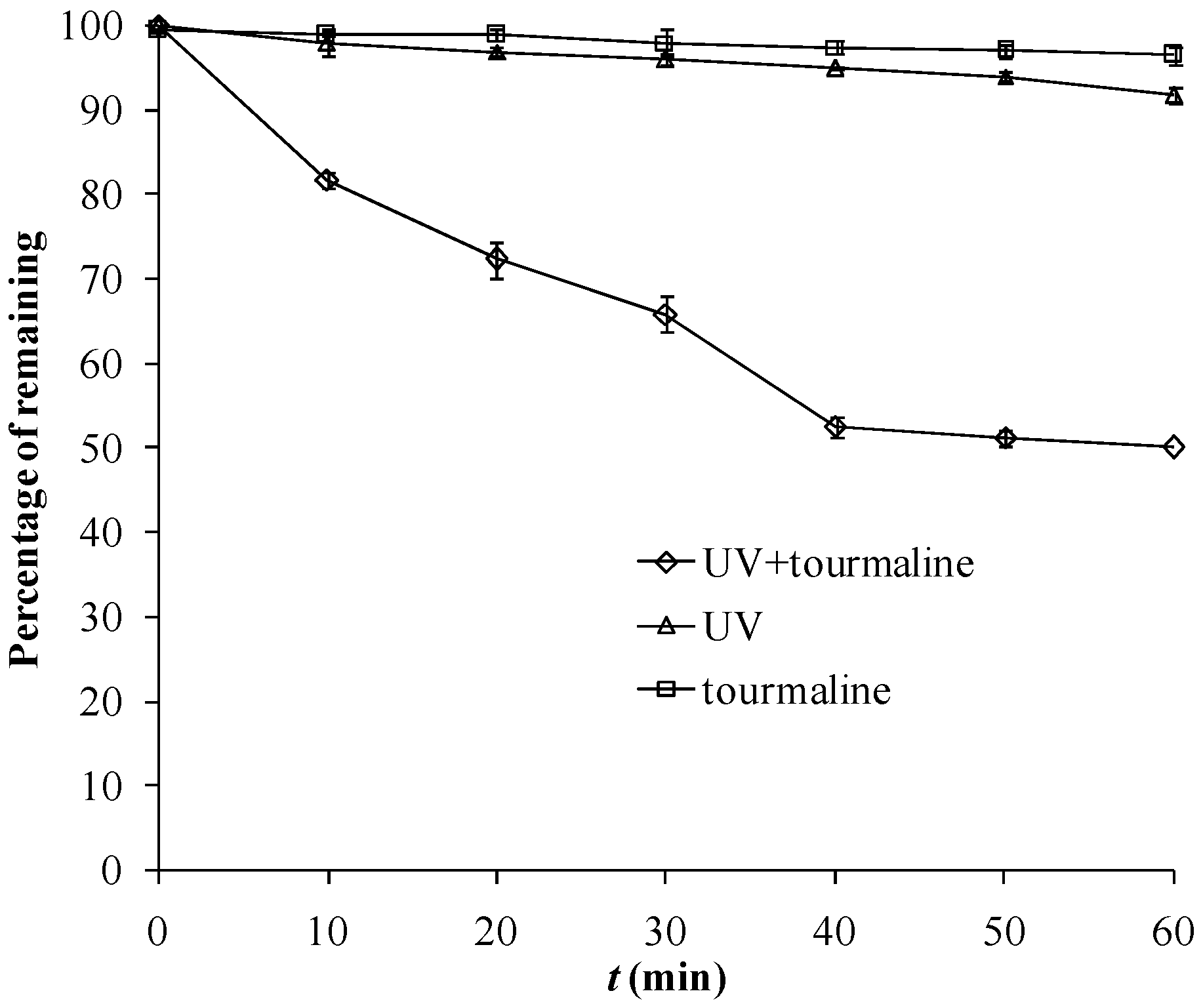
2.2. Effect of Ethanol

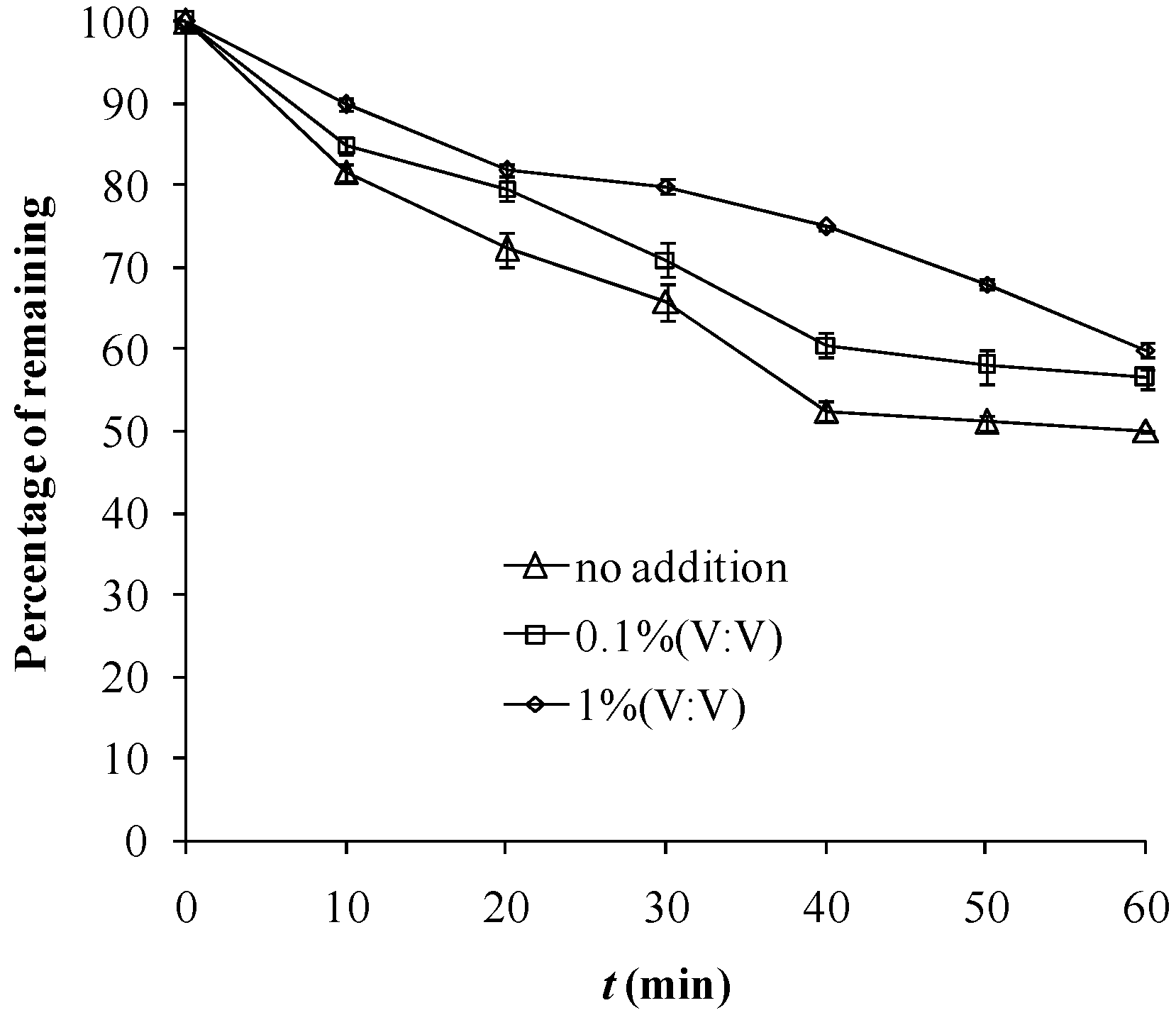
2.3. Effect of Cl− and SO42−


2.4. Effect of Cu2+ and Mg2+
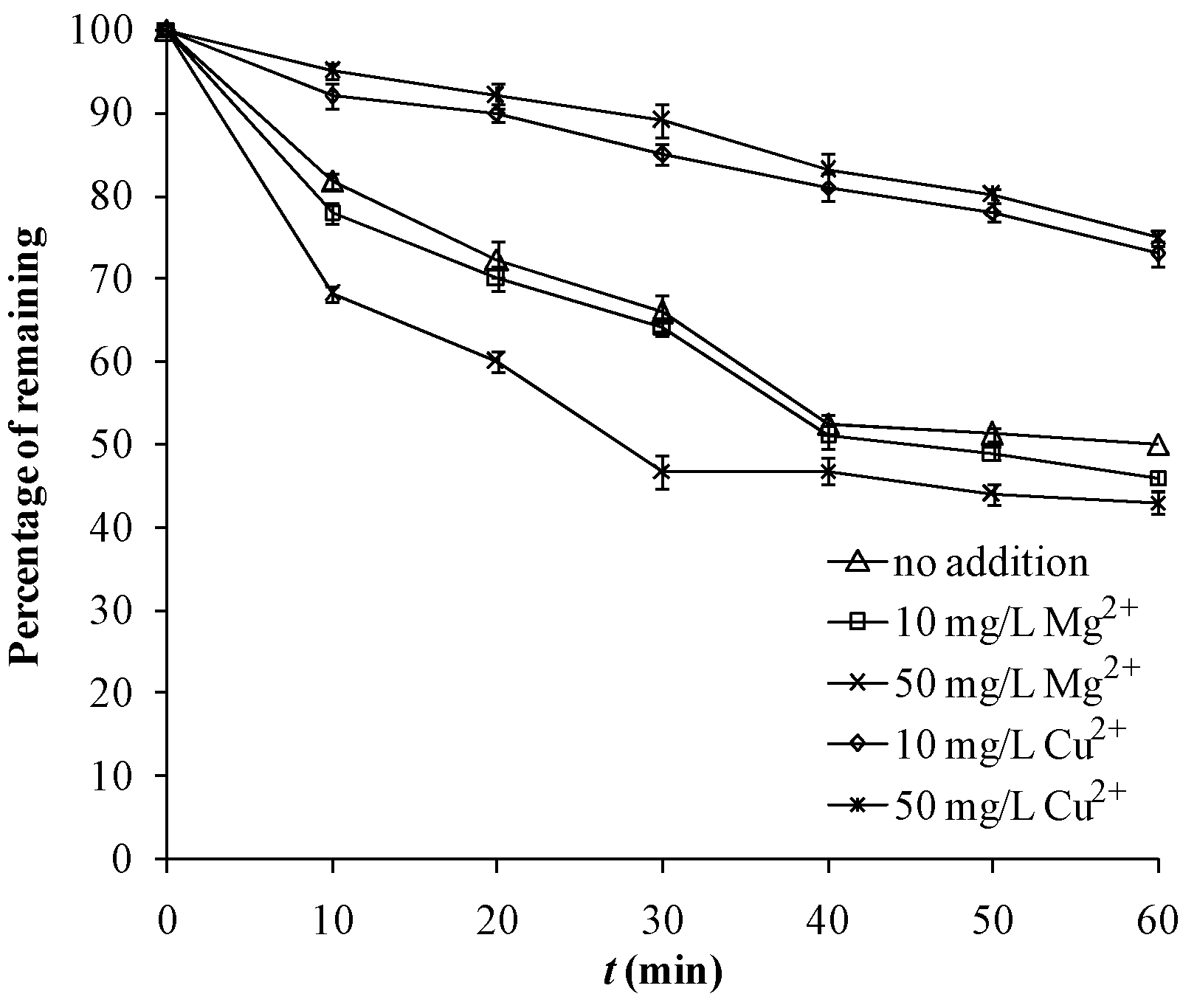
3. Experimental
3.1. General
3.2. Photocatalytic Degradation of MB by Crude Fe-Tourmaline
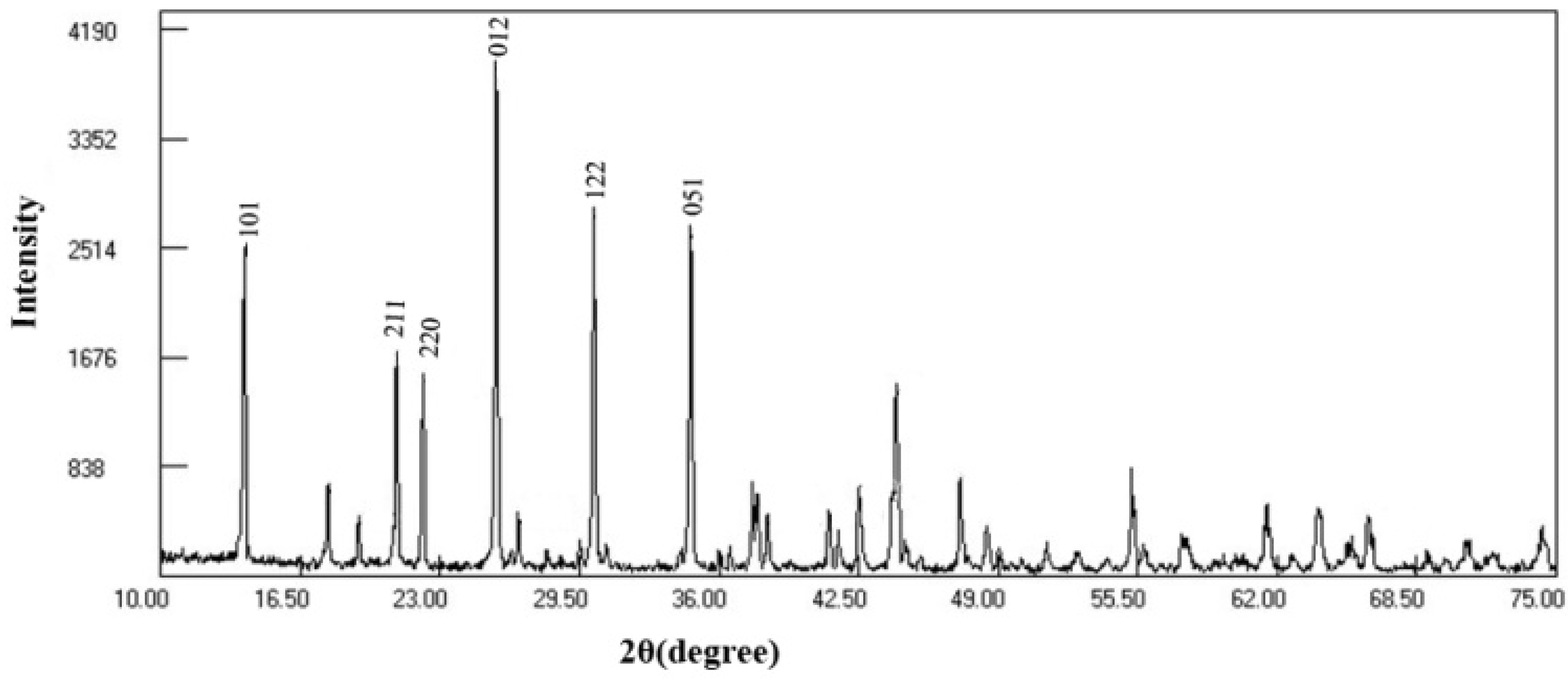
3.3. HPLC Analysis and Tourmaline Characterization
4. Conclusions
- Sample Availability: Samples of the compounds are available from the authors.
References
- Hessel, C.; Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007, 83, 171–180. [Google Scholar] [CrossRef]
- Nigam, P.; Armour, G.; Banat, I.-M.; Singh, D.; Marchant, R. Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agricultural residues. Bioresour. Technol. 2000, 72, 219–226. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Saggioro, E.M.; Oliveira, A.S.; Pavesi, T.; Maia, C.G.; Ferreira, L.F.V.; Moreira, J.C. Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 2011, 16, 10370–10386. [Google Scholar] [CrossRef]
- Peng, X.-Y.; Feng, L.; Liu, L. Synergistic degradation of dyeing wastewater by the ultrasound/Fenton method. J. Beijing Univ. Chem. Technol. (Nat. Sci. Ed.) 2007, 34, 122–125. (in Chinese).. [Google Scholar]
- Šojić, D.; Despotović, V.; Abramović, B.; Todorova, N.; Giannakopoulou, T.; Trapalis, C. Photocatalytic degradation of mecoprop and clopyralid in aqueous suspensions of nanostructured N-doped TiO2. Molecules 2010, 15, 2994–3009. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, R.; Zhang, X. The mechanism of applying tourmaline to purifying Cu2+-doped waste water. Acta Petrol Miner. 2002, 21, 192–196. [Google Scholar]
- Ji, Z.; Jin, Z.; Liang, J.; Wang, J.; Yan, X. Influence of tourmaline on pH value of water. Chin. Environ. Sci. 2002, 22, 515–519. [Google Scholar]
- Xu, H.; Prasad, M.; Liu, Y. Schorl: A novel catalyst in mineral-catalyzed Fenton-like system for dyeing wastewater discoloration. J. Hazard. Mater. 2009, 165, 1186–1192. [Google Scholar] [CrossRef]
- Tokumura, M.; Znad, H.-T.; Kawase, Y. Modeling of an external light irradiation slurry photoreactor: UV light or sunlight-photoassisted Fenton discoloration of azo-dye Orange II with natural mineral tourmaline powder. Chem. Eng. Sci. 2006, 61, 6361–6371. [Google Scholar] [CrossRef]
- Meng, J.; Liang, J.; Ou, X.; Ding, Y.; Liang, G. Effects of mineral tourmaline particles on the photocatalytic activity of TiO2 thin films. J. Nanosci. Nanotechnol. 2008, 8, 1279–1283. [Google Scholar]
- Du, W.; Xu, Y.; Wang, Y. Photoinduced degradation of orange II on different iron (hydr)oxides in aqueous suspension: Rate enhancement on addition of hydrogen peroxide, silver nitrate, and sodium fluoride. Langmuir 2008, 24, 175–181. [Google Scholar] [CrossRef]
- Yan, X.; Bao, R.; Yu, S.; Li, Q.; Jing, Q. The roles of hydroxyl radicals, photo-generated holes and oxygen in the photocatalytic degradation of humic acid. Russ. J. Phys. Chem. A 2012, 86, 1479–1485. [Google Scholar]
- Gondal, M.-A.; Sayeed, M.-N.; Alarfaj, A. Activity comparison of Fe2O3, NiO, WO3, TiO2 semiconductor catalysts in phenol degradation by laser enhanced photo-catalytic process. Chem. Phys. Lett. 2007, 445, 325–330. [Google Scholar] [CrossRef]
- Pal, B.; Sharon, M. Photocatalytic degradation of salicylic acid by colloidal Fe2O3 particles. Chem. Phys. Lett. 2007, 73, 269–273. [Google Scholar]
- Abdullah, M.; Low, G.-K.-C.; Matthews, R.-W. Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide. J. Phys. Chem. 1990, 94, 6820–6825. [Google Scholar] [CrossRef]
- Wang, K.-H.; Hsieh, Y.-H.; Wu, C.-H.; Chang, C.-Y. The pH and anion effects on the heterogeneous photocatalytic degradation of o-methylbenzoic acid in TiO2 aqueous suspension. Chemosphere 2000, 40, 389–394. [Google Scholar] [CrossRef]
- Litter, M.-I. Heterogeneous photocatalysis transition metal ions in photocatalytic systems. Appl. Catal. B 1999, 23, 89–114. [Google Scholar]
- Okamoto, K.; Yamamoto, Y.; Tanaka, H.; Tanaka, M.; Itaya, A. Heterogeneous photocatalytic decomposition of phenol over TiO2 powder. Bull. Chem. Soc. Jpn. 1985, 58, 2015–2022. [Google Scholar] [CrossRef]
- Jiang, F.; Zheng, Z.; Xu, Z.; Zheng, S. Preparation and characterization of SiO2-pillared H2Ti4O9 and its photocatalytic activity for methylene blue degradation. J. Hazard. Mater. 2009, 164, 1250–1256. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bian, X.; Chen, J.; Ji, R. Degradation of Methyl Blue Using Fe-Tourmaline as a Novel Photocatalyst. Molecules 2013, 18, 1457-1463. https://doi.org/10.3390/molecules18021457
Bian X, Chen J, Ji R. Degradation of Methyl Blue Using Fe-Tourmaline as a Novel Photocatalyst. Molecules. 2013; 18(2):1457-1463. https://doi.org/10.3390/molecules18021457
Chicago/Turabian StyleBian, Xuesen, Jianqiu Chen, and Rong Ji. 2013. "Degradation of Methyl Blue Using Fe-Tourmaline as a Novel Photocatalyst" Molecules 18, no. 2: 1457-1463. https://doi.org/10.3390/molecules18021457



